Theoretical studies of structure, function and reactivity of molecules--a personal account
- PMID: 19444009
- PMCID: PMC3524299
- DOI: 10.2183/pjab.85.167
Theoretical studies of structure, function and reactivity of molecules--a personal account
Abstract
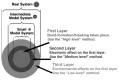
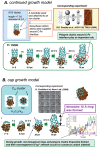
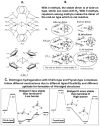
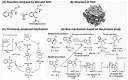
Last few decades theoretical/computational studies of structure, function and reactivity of molecules have been contributing significantly in chemistry by explanation of experimental results, better understanding of underlying principles and prediction of the unknown experimental outcome. Accuracy needed in chemistry has long been established, but due to high power dependency of such accurate methods on the molecular size, it has been a major challenge to apply theoretical methods to large molecular systems. In the present article we will review some examples of such applications. One is theoretical study of growth/formation of carbon nanostructures such as fullerenes and carbon nanotubes, using quantum mechanical molecular dynamics method. For growth of single walled carbon nanotube from transition metal cluster, we have demonstrated continued growth of attached nanotube, cap formation and growth from small carbon fragments. For homogeneous catalysis we presented results of studies on N(2) activation by Zr complexes. For biomolecular reactions we use active site and protein models and show that in some catalyses the protein environment is involved in reactions and changes the preferred pathway, and in some other case the effect is modest. The review is concluded with a perspective.
Figures





Similar articles
-
Atomistic simulations of catalyzed carbon nanotube growth.J Nanosci Nanotechnol. 2006 May;6(5):1211-24. doi: 10.1166/jnn.2006.145. J Nanosci Nanotechnol. 2006. PMID: 16792348 Review.
-
Fe/C interactions during SWNT growth with C2 feedstock molecules: A quantum chemical molecular dynamics study.J Nanosci Nanotechnol. 2006 May;6(5):1259-70. doi: 10.1166/jnn.2006.142. J Nanosci Nanotechnol. 2006. PMID: 16792352
-
Hybrid schemes based on quantum mechanics/molecular mechanics simulations goals to success, problems, and perspectives.Adv Protein Chem Struct Biol. 2011;85:81-142. doi: 10.1016/B978-0-12-386485-7.00003-X. Adv Protein Chem Struct Biol. 2011. PMID: 21920322 Review.
-
The importance of strong carbon-metal adhesion for catalytic nucleation of single-walled carbon nanotubes.Nano Lett. 2008 Feb;8(2):463-8. doi: 10.1021/nl072431m. Epub 2007 Dec 28. Nano Lett. 2008. PMID: 18162001
-
Changes in single-walled carbon nanotube chirality during growth and regrowth.J Chem Phys. 2008 Mar 28;128(12):124708. doi: 10.1063/1.2876464. J Chem Phys. 2008. PMID: 18376961
Cited by
-
Molecular insight on the non-covalent interactions between carbapenems and L,D-transpeptidase 2 from Mycobacterium tuberculosis: ONIOM study.J Comput Aided Mol Des. 2018 Jun;32(6):687-701. doi: 10.1007/s10822-018-0121-2. Epub 2018 May 29. J Comput Aided Mol Des. 2018. PMID: 29845435
References
-
- Maseras F., Morokuma K. (1995) IMOMM: A new integrated ab initio + molecular mechanics geometry optimization scheme of equilibrium structures and transition states. J. Comp. Chem. 16, 1170–1179
-
- Svensson M., Humbel S., Froese R. D. J., Matsubara T., Sieber A., Morokuma K. (1996) ONIOM: A multilayered Integrated MO + MM method for geometry optimizations and single point energy predictions. A test for Diels–Alder re- actions and Pt(P(t-Bu)3)2 + H2 oxidative addition. J. Phys. Chem. 100, 19357–19363
-
- Dapprich S., Komaromi I., Byun K. S., Morokuma K., Frisch M. J. (1999) A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Str. (Theochem) 461–462, 1–21
-
- Vreven T., Morokuma K. (2000) On the application of the IMOMO (integrated molecular orbital + molecular orbital) method. J. Comp. Chem. 21, 1419–1432
-
- Morokuma K. (2003) ONIOM and its applications to material chemistry and catalyses. Bull. Kor. Chem. Soc. 24, 797–801
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

