Autoimmune T cell responses in the central nervous system
- PMID: 19444307
- PMCID: PMC2813731
- DOI: 10.1038/nri2550
Autoimmune T cell responses in the central nervous system
Abstract
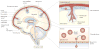
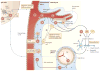
Autoreactive T cell responses have a crucial role in central nervous system (CNS) diseases such as multiple sclerosis. Recent data indicate that CNS autoimmunity can be mediated by two distinct lineages of CD4+ T cells that are defined by the production of either interferon-gamma or interleukin-17. The activity of these CD4+ T cell subsets within the CNS influences the pathology and clinical course of disease. New animal models show that myelin-specific CD8+ T cells can also mediate CNS autoimmunity. This Review focuses on recent progress in delineating the pathogenic mechanisms, regulation and interplay between these different T cell subsets in CNS autoimmunity.
Figures


References
-
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nature Immunol. 2007;8:913–919. - PubMed
-
- Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. - PubMed
-
- Raine C. In: Multiple Sclerosis: Clinical and Pathogenetic Basis. Raine CS, McFarland HF, Tourtellotte WW, editors. Chapman and Hall; London: 1997. pp. 243–286.
-
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nature Rev Immunol. 2003;3:569–581. - PubMed
-
- Huseby ES, Sather B, Huseby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

