In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes
- PMID: 19444330
- PMCID: PMC2681269
- DOI: 10.1177/193229680900300106
In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes
Abstract
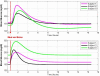
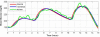
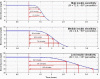
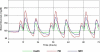
Arguably, a minimally invasive system using subcutaneous (s.c.) continuous glucose monitoring (CGM) and s.c. insulin delivery via insulin pump would be a most feasible step to closed-loop control in type 1 diabetes mellitus (T1DM). Consequently, diabetes technology is focusing on developing an artificial pancreas using control algorithms to link CGM with s.c. insulin delivery. The future development of the artificial pancreas will be greatly accelerated by employing mathematical modeling and computer simulation. Realistic computer simulation is capable of providing invaluable information about the safety and the limitations of closed-loop control algorithms, guiding clinical studies, and out-ruling ineffective control scenarios in a cost-effective manner. Thus computer simulation testing of closed-loop control algorithms is regarded as a prerequisite to clinical trials of the artificial pancreas. In this paper, we present a system for in silico testing of control algorithms that has three principal components: (1) a large cohort of n=300 simulated "subjects" (n=100 adults, 100 adolescents, and 100 children) based on real individuals' data and spanning the observed variability of key metabolic parameters in the general population of people with T1DM; (2) a simulator of CGM sensor errors representative of Freestyle Navigator™, Guardian RT, or Dexcom™ STS™, 7-day sensor; and (3) a simulator of discrete s.c. insulin delivery via OmniPod Insulin Management System or Deltec Cozmo(®) insulin pump. The system has been shown to represent adequate glucose fluctuations in T1DM observed during meal challenges, and has been accepted by the Food and Drug Administration as a substitute to animal trials in the preclinical testing of closed-loop control strategies.
Keywords: computer simulation; diabetes control; modeling.
© Diabetes Technology Society
Figures
References
-
- Pfeiffer EF, Thum C, Clemens AH. The artificial beta cell—a continuous control of blood sugar by external regulation of insulin infusion (glucose controlled insulin infusion system) Horm Metab Res. 1974;6(5):339–342. - PubMed
-
- Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W. An artificial endocrine pancreas. Diabetes. 1974;23(5):389–396. - PubMed
-
- Clemens AH, Chang PH, Myers RW. The development of Biostator, a glucose-controlled insulin infusion system. Horm Metab Res. 1977;(Suppl 7):23–33. - PubMed
-
- Marliss EB, Murray FT, Stokes EF, Zinman B, Nakhooda AF, Denoga A, Leibel BS, Albisser AM. Normalization of glycemia in diabetics during meals with insulin and glucagon delivery by the artificial pancreas. Diabetes. 1977;26(7):663–672. - PubMed
-
- Santiago JV, Clemens AH, Clarke WL, Kipnis DM. Closed-loopand open-loop devices for blood glucose control in normal and diabetic subjects. Diabetes. 1979;28(1):71–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

