Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation
- PMID: 19444869
- PMCID: PMC2738985
- DOI: 10.1002/hep.22926
Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation
Abstract
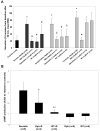
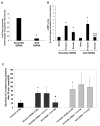
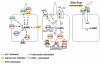
Cyclic adenosine monophosphate (cAMP) is generated by adenylyl cyclases (ACs), a group of enzymes with different tissue specificity and regulation. We hypothesized that AC isoforms are heterogeneously expressed along the biliary tree, are associated with specific secretory stimuli, and are differentially modulated in cholestasis. Small duct and large duct cholangiocytes were isolated from controls and from lipopolysaccharide-treated or alpha-naphthylisothiocyanate-treated rats. AC isoform expression was assessed via real-time polymerase chain reaction. Secretion and cAMP levels were measured in intrahepatic bile duct units after stimulation with secretin, forskolin, HCO(3)(-)/CO(2), cholinergic agonists, and beta-adrenergic agonists, with or without selected inhibitors or after silencing of AC8 or soluble adenylyl cyclase (sAC) with small interfering RNA. Gene expression of the Ca(2+)-insensitive isoforms (AC4, AC7) was higher in small duct cholangiocytes, whereas that of the Ca(2+)-inhibitable (AC5, AC6, AC9), the Ca(2+)/calmodulin-stimulated AC8, and the soluble sAC was higher in large duct cholangiocytes. Ca(2+)/calmodulin inhibitors and AC8 gene silencing inhibited choleresis and cAMP production stimulated by secretin and acetylcholine, but not by forskolin. Secretion stimulated by isoproterenol and calcineurin inibitors was cAMP-dependent and gamma-aminobutyric acid-inhibitable, consistent with activation of AC9. Cholangiocyte secretion stimulated by isohydric changes in [HCO(3)(-)](i) was cAMP-dependent and inhibited by sAC inhibitor and sAC gene silencing. Treatment with lipopolysaccharide or alpha-naphthylisothiocyanate increased expression of AC7 and sAC but decreased expression of the other ACs.
Conclusion: These studies demonstrate a previously unrecognized role of ACs in biliary pathophysiology. In fact: (1) AC isoforms are differentially expressed in cholangiocyte subpopulations; (2) AC8, AC9, and sAC mediate cholangiocyte secretion in response to secretin, beta-adrenergic agonists, or changes in [HCO(3)(-)](i), respectively; and (3) AC gene expression is modulated in experimental cholestasis.
Figures



References
-
- Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. - PubMed
-
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
-
- Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–1074. - PubMed
-
- Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
