Posttranslational regulation of Myc by promyelocytic leukemia zinc finger protein
- PMID: 19444914
- PMCID: PMC2721905
- DOI: 10.1002/ijc.24449
Posttranslational regulation of Myc by promyelocytic leukemia zinc finger protein
Abstract
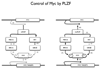
The promyelocytic leukemia zinc finger (PLZF) protein, a transcriptional repressor, induces cellular resistance to oncogenic transformation by diverse oncoproteins. Two point mutants of PLZF that have lost the antioncogenic activity of the wild-type protein are oncogenic in chicken embryo fibroblasts; this activity is correlated with differential effects on Myc. Wild-type PLZF represses Myc transcription without affecting total Myc protein levels and reduces the levels of phosphorylated Myc. The PLZF mutants do not alter Myc transcription or protein expression but increase the levels of phosphorylated Myc. These modifications of Myc are correlated with PLZF-induced changes in Akt and the mitogen-activated protein kinase (MAPK) pathway. Wild-type PLZF downregulates the MAPK pathway and activates Akt, resulting in reduced phosphorylation on serine 62 of Myc by Erk and on threonine 58 by glycogen synthase kinase 3beta. The mutants fail to activate Akt and only slightly downregulate phospho-Erk. We postulate that the 2 PLZF mutants are oncogenic, because they function as dominant negatives of wild-type PLZF, enhancing Myc phosphorylation and increasing Myc transcriptional and oncogenic activity. In support of this suggestion, a specific inhibitor of Myc is able to revert the transformed phenotype of PLZF mutant-expressing cells.
Figures



Similar articles
-
AML-1/ETO fusion protein is a dominant negative inhibitor of transcriptional repression by the promyelocytic leukemia zinc finger protein.Blood. 2000 Dec 1;96(12):3939-47. Blood. 2000. PMID: 11090081
-
Role of promyelocytic leukemia zinc finger (PLZF) in cell proliferation and cyclin-dependent kinase inhibitor 1A (p21WAF/CDKN1A) gene repression.J Biol Chem. 2014 Jul 4;289(27):18625-40. doi: 10.1074/jbc.M113.538751. Epub 2014 May 12. J Biol Chem. 2014. PMID: 24821727 Free PMC article.
-
Comprehensive genomic screens identify a role for PLZF-RARalpha as a positive regulator of cell proliferation via direct regulation of c-MYC.Blood. 2009 Dec 24;114(27):5499-511. doi: 10.1182/blood-2009-03-206524. Epub 2009 Oct 23. Blood. 2009. PMID: 19855079 Free PMC article.
-
Promyelocytic leukemia zinc finger controls type 2 immune responses in the lungs by regulating lineage commitment and the function of innate and adaptive immune cells.Int Immunopharmacol. 2024 Mar 30;130:111670. doi: 10.1016/j.intimp.2024.111670. Epub 2024 Feb 18. Int Immunopharmacol. 2024. PMID: 38373386 Review.
-
The PLZF gene of t (11;17)-associated APL.Curr Top Microbiol Immunol. 2007;313:31-48. doi: 10.1007/978-3-540-34594-7_3. Curr Top Microbiol Immunol. 2007. PMID: 17217037 Review.
Cited by
-
PLZF, a tumor suppressor genetically lost in metastatic castration-resistant prostate cancer, is a mediator of resistance to androgen deprivation therapy.Cancer Res. 2015 May 15;75(10):1944-8. doi: 10.1158/0008-5472.CAN-14-3602. Epub 2015 Mar 25. Cancer Res. 2015. PMID: 25808865 Free PMC article.
-
Smooth muscle α-actin is a direct target of PLZF: effects on the cytoskeleton and on susceptibility to oncogenic transformation.Oncotarget. 2010 May;1(1):9-21. doi: 10.18632/oncotarget.104. Oncotarget. 2010. PMID: 20634973 Free PMC article.
-
Crosstalk Between MYC and lncRNAs in Hematological Malignancies.Front Oncol. 2020 Oct 8;10:579940. doi: 10.3389/fonc.2020.579940. eCollection 2020. Front Oncol. 2020. PMID: 33134177 Free PMC article. Review.
-
MYC-repressed long noncoding RNAs antagonize MYC-induced cell proliferation and cell cycle progression.Oncotarget. 2015 Aug 7;6(22):18780-9. doi: 10.18632/oncotarget.3909. Oncotarget. 2015. PMID: 26003165 Free PMC article.
-
Distinct signal transduction pathways downstream of the (P)RR revealed by microarray and ChIP-chip analyses.PLoS One. 2013;8(3):e57674. doi: 10.1371/journal.pone.0057674. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23469216 Free PMC article.
References
-
- Chen SJ, Zelent A, Tong JH, Yu HQ, Wang ZY, Derre J, Berger R, Waxman S, Chen Z. Rearrangements of the retinoic acid receptor alpha and promyelocytic leukemia zinc finger genes resulting from t(11;17)(q23;q21) in a patient with acute promyelocytic leukemia. J Clin Invest. 1993;91:2260–2267. - PMC - PubMed
-
- Li JY, English MA, Ball HJ, Yeyati PL, Waxman S, Licht JD. Sequence-specific DNA binding and transcriptional regulation by the promyelocytic leukemia zinc finger protein. J Biol Chem. 1997;272:22447–22455. - PubMed
-
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

