Osmolality-induced tuning of action potentials in trigeminal ganglion neurons
- PMID: 19444958
- PMCID: PMC2775522
- DOI: 10.1016/j.neulet.2009.01.022
Osmolality-induced tuning of action potentials in trigeminal ganglion neurons
Abstract
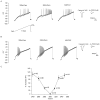
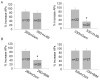
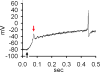
The present study explored the effect of anisotonicity on action potential (AP) in cultured trigeminal ganglion (TG) neurons. We demonstrate that the number of evoked APs was increased by both hypo- and hypertonic treatment. Transient Receptor Potential Vanilloid 4 receptor (TRPV4) activator increased the number of APs, but only hypotonic-response was markedly blocked in TRPV4-/- mice. Additionally, inhibition of PKC attenuated hypotonicity-induced increase, whereas antagonism of PKA attenuated hypertonicity-response. We conclude that anisotonicity increases excitability of nociceptors, which might be involved in anisotonicity-induced nociception. The increase of APs by hypo- and hypertonicity is mediated through different receptor and intracellular signaling pathways.
Figures
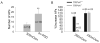
Similar articles
-
Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons.Brain Res. 2008 May 7;1208:56-66. doi: 10.1016/j.brainres.2008.02.048. Epub 2008 Feb 29. Brain Res. 2008. PMID: 18378217 Free PMC article.
-
The modulation of voltage-gated potassium channels by anisotonicity in trigeminal ganglion neurons.Neuroscience. 2008 Jun 23;154(2):482-95. doi: 10.1016/j.neuroscience.2008.03.046. Epub 2008 Mar 29. Neuroscience. 2008. PMID: 18456412 Free PMC article.
-
Changes in osmolality modulate voltage-gated sodium channels in trigeminal ganglion neurons.Neurosci Res. 2009 Jun;64(2):199-207. doi: 10.1016/j.neures.2009.02.012. Epub 2009 Mar 13. Neurosci Res. 2009. PMID: 19428701 Free PMC article.
-
Changes in osmolality sensitize the response to capsaicin in trigeminal sensory neurons.J Neurophysiol. 2007 Mar;97(3):2001-15. doi: 10.1152/jn.00887.2006. J Neurophysiol. 2007. PMID: 17353553
-
Hypotonicity modulates tetrodotoxin-sensitive sodium current in trigeminal ganglion neurons.Mol Pain. 2011 Apr 16;7:27. doi: 10.1186/1744-8069-7-27. Mol Pain. 2011. PMID: 21496300 Free PMC article.
Cited by
-
Hyperosmolar tears enhance cooling sensitivity of the corneal nerves in rats: possible neural basis for cold-induced dry eye pain.Invest Ophthalmol Vis Sci. 2014 Aug 19;55(9):5821-33. doi: 10.1167/iovs.14-14642. Invest Ophthalmol Vis Sci. 2014. PMID: 25139732 Free PMC article.
-
Transient Receptor Potential Vanilloid 4-Induced Modulation of Voltage-Gated Sodium Channels in Hippocampal Neurons.Mol Neurobiol. 2016 Jan;53(1):759-768. doi: 10.1007/s12035-014-9038-5. Epub 2014 Dec 15. Mol Neurobiol. 2016. PMID: 25502461
-
AAV-mediated gene therapy targeting TRPV4 mechanotransduction for inhibition of pulmonary vascular leakage.APL Bioeng. 2019 Dec 2;3(4):046103. doi: 10.1063/1.5122967. eCollection 2019 Dec. APL Bioeng. 2019. PMID: 31803860 Free PMC article.
-
Avian magnetite-based magnetoreception: a physiologist's perspective.J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S193-205. doi: 10.1098/rsif.2009.0423.focus. Epub 2010 Jan 27. J R Soc Interface. 2010. PMID: 20106875 Free PMC article.
-
Association Between Serum Sodium and Long-Term Mortality in Critically Ill Patients with Comorbid Chronic Obstructive Pulmonary Disease: Analysis from the MIMIC-IV Database.Int J Chron Obstruct Pulmon Dis. 2022 May 12;17:1143-1155. doi: 10.2147/COPD.S353741. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 35586119 Free PMC article.
References
-
- Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32:275–285. - PubMed
-
- Rosen AS, Andrew RD. Osmotic effects upon excitability in rat neocortical slices. Neuroscience. 1990;38:579–590. - PubMed
-
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. - PubMed
-
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

