DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival
- PMID: 19446321
- PMCID: PMC2758791
- DOI: 10.1016/j.cell.2009.03.046
DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival
Abstract
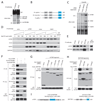
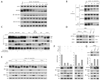
The mTORC1 and mTORC2 pathways regulate cell growth, proliferation, and survival. We identify DEPTOR as an mTOR-interacting protein whose expression is negatively regulated by mTORC1 and mTORC2. Loss of DEPTOR activates S6K1, Akt, and SGK1, promotes cell growth and survival, and activates mTORC1 and mTORC2 kinase activities. DEPTOR overexpression suppresses S6K1 but, by relieving feedback inhibition from mTORC1 to PI3K signaling, activates Akt. Consistent with many human cancers having activated mTORC1 and mTORC2 pathways, DEPTOR expression is low in most cancers. Surprisingly, DEPTOR is highly overexpressed in a subset of multiple myelomas harboring cyclin D1/D3 or c-MAF/MAFB translocations. In these cells, high DEPTOR expression is necessary to maintain PI3K and Akt activation and a reduction in DEPTOR levels leads to apoptosis. Thus, we identify a novel mTOR-interacting protein whose deregulated overexpression in multiple myeloma cells represents a mechanism for activating PI3K/Akt signaling and promoting cell survival.
Figures




References
-
- Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans. 2001;29:1–14. - PubMed
-
- Ballon DR, Flanary PL, Gladue DP, Konopka JB, Dohlman HG, Thorner J. DEP-domain-mediated regulation of GPCR signaling responses. Cell. 2006;126:1079–1093. - PubMed
-
- Bliskovsky V, Ramsay ES, Scott J, DuBois W, Shi W, Zhang S, Qian X, Lowy DR, Mock BA. Frap, FKBP12 rapamycin-associated protein, is a candidate gene for the plasmacytoma resistance locus Pctr2 and can act as a tumor suppressor gene. Proc Natl Acad Sci U S A. 2003;100:14982–14987. Epub 12003 Nov 14921. - PMC - PubMed
-
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

