Gene therapy of benign gynecological diseases
- PMID: 19446586
- PMCID: PMC4477532
- DOI: 10.1016/j.addr.2009.04.023
Gene therapy of benign gynecological diseases
Abstract
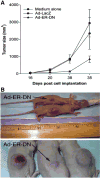
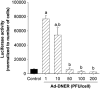
Gene therapy is the introduction of genetic material into patient's cells to achieve therapeutic benefit. Advances in molecular biology techniques and better understanding of disease pathogenesis have validated the use of a variety of genes as potential molecular targets for gene therapy based approaches. Gene therapy strategies include: mutation compensation of dysregulated genes; replacement of defective tumor-suppressor genes; inactivation of oncogenes; introduction of suicide genes; immunogenic therapy and antiangiogenesis based approaches. Preclinical studies of gene therapy for various gynecological disorders have not only shown to be feasible, but also showed promising results in diseases such as uterine leiomyomas and endometriosis. In recent years, significant improvement in gene transfer technology has led to the development of targetable vectors, which have fewer side-effects without compromising their efficacy. This review provides an update on developing gene therapy approaches to treat common gynecological diseases such as uterine leiomyoma and endometriosis.
Figures





Similar articles
-
Report of the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development-sponsored workshop: gynecology and women's health-benign conditions and cancer.Am J Obstet Gynecol. 2020 Dec;223(6):796-808. doi: 10.1016/j.ajog.2020.08.049. Epub 2020 Aug 21. Am J Obstet Gynecol. 2020. PMID: 32835714
-
Gene transfer approaches for gynecological diseases.Mol Ther. 2006 Aug;14(2):154-63. doi: 10.1016/j.ymthe.2006.02.019. Epub 2006 May 2. Mol Ther. 2006. PMID: 16650808 Review.
-
MicroRNA and gynecological reproductive diseases.Fertil Steril. 2014 Jun;101(6):1545-51. doi: 10.1016/j.fertnstert.2014.04.044. Fertil Steril. 2014. PMID: 24882618 Review.
-
Report on a conference on developments in the treatment of benign gynecological disorders.Gynecol Endocrinol. 1992 Dec;6(4):301-9. doi: 10.3109/09513599209024995. Gynecol Endocrinol. 1992. PMID: 1492588 No abstract available.
-
Role of microRNAs in gynecological pathology.Curr Med Chem. 2012;19(15):2406-13. doi: 10.2174/092986712800269362. Curr Med Chem. 2012. PMID: 22455593 Review.
Cited by
-
Gene Therapy for Malignant and Benign Gynaecological Disorders: A Systematic Review of an Emerging Success Story.Cancers (Basel). 2022 Jun 30;14(13):3238. doi: 10.3390/cancers14133238. Cancers (Basel). 2022. PMID: 35805007 Free PMC article. Review.
-
Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations.Hum Reprod Update. 2014 May-Jun;20(3):309-33. doi: 10.1093/humupd/dmt058. Epub 2014 Jan 8. Hum Reprod Update. 2014. PMID: 24401287 Free PMC article.
-
Bacillus coagulans TBC169 probiotics for the recovery of intestinal function after gynecological laparoscopic surgery: a randomized, placebo-controlled trial.Int J Clin Pharm. 2022 Dec;44(6):1287-1295. doi: 10.1007/s11096-022-01451-2. Epub 2022 Jul 27. Int J Clin Pharm. 2022. PMID: 35882823 Clinical Trial.
-
Targeted Adenoviral Vector Demonstrates Enhanced Efficacy for In Vivo Gene Therapy of Uterine Leiomyoma.Reprod Sci. 2016 Apr;23(4):464-74. doi: 10.1177/1933719116630413. Epub 2016 Feb 16. Reprod Sci. 2016. PMID: 26884457 Free PMC article.
-
Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study.Int J Womens Health. 2013;5:93-100. doi: 10.2147/IJWH.S38800. Epub 2013 Feb 27. Int J Womens Health. 2013. PMID: 23467803 Free PMC article.
References
-
- Trent RJA, Alexander IE. Clinical perspectives gene therapy: applications and progress towards the clinic. Int Med J. 2004;34:621–625. - PubMed
-
- Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475–480. - PubMed
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. - PubMed
-
- Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review, Hum. Reprod Updat. 2006;12:385–400. - PubMed
-
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;2:91–99. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

