Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein
- PMID: 19446590
- PMCID: PMC2741729
- DOI: 10.1016/j.vaccine.2009.05.008
Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein
Abstract
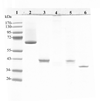
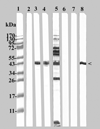
To compare the ability of a native and a recombinant preparation of the major outer membrane protein of Chlamydia trachomatis mouse pneumonitis (MoPn; Ct-nMOMP and Ct-rMOMP) to protect against an intranasal (i.n.) challenge, BALB/c mice were vaccinated by the intramuscular (i.m.) and subcutaneous (s.c.) routes using CpG-1826 and Montanide ISA 720 as adjuvants. Animals inoculated i.n. with live elementary bodies (EB) of Chlamydia served as a positive control. Negative control groups were immunized with either Neisseria gonorrhoeae recombinant porin B (Ng-rPorB) or with minimal essential medium (MEM-0). Mice immunized with Ct-rMOMP, Ct-nMOMP and EB developed a strong immune response as shown by high levels of Chlamydia specific antibodies in serum and a strong T-cell lymphoproliferative response. Following the i.n. challenge with 10(4) inclusion forming units (IFU) of C. trachomatis, mice immunized with Ct-nMOMP or Ct-rMOMP lost significantly less weight than the negative control animals immunized with Ng-rPorB or MEM-0 (P<0.05). However, mice vaccinated with the Ct-nMOMP lost less weight than those immunized with the Ct-rMOMP (P<0.05). Mice were euthanized at 10 days following the challenge, their lungs weighed and the number of IFU of Chlamydia determined. Based on the lung weight and number of IFU recovered, significant protection was observed in the groups of mice immunized with both Ct-nMOMP and the Ct-rMOMP (P<0.05). Nevertheless, significantly better protection was achieved with the Ct-nMOMP in comparison with the Ct-rMOMP (P<0.05). In conclusion, vaccination with a preparation of the nMOMP elicited a more robust protection than immunization with rMOMP, suggesting that the conformational structure of MOMP is critical for inducing strong protection.
Figures

Similar articles
-
Vaccination with the recombinant major outer membrane protein elicits antibodies to the constant domains and induces cross-serovar protection against intranasal challenge with Chlamydia trachomatis.Infect Immun. 2013 May;81(5):1741-50. doi: 10.1128/IAI.00734-12. Epub 2013 Mar 11. Infect Immun. 2013. PMID: 23478318 Free PMC article.
-
The cationic liposomal adjuvants CAF01 and CAF09 formulated with the major outer membrane protein elicit robust protection in mice against a Chlamydia muridarum respiratory challenge.Vaccine. 2017 Mar 23;35(13):1705-1711. doi: 10.1016/j.vaccine.2017.02.020. Epub 2017 Feb 24. Vaccine. 2017. PMID: 28238632
-
A TLR2 agonist is a more effective adjuvant for a Chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors.Vaccine. 2011 Sep 2;29(38):6641-9. doi: 10.1016/j.vaccine.2011.06.105. Epub 2011 Jul 8. Vaccine. 2011. PMID: 21742006 Free PMC article.
-
Enhancement of the protective efficacy of a Chlamydia trachomatis recombinant vaccine by combining systemic and mucosal routes for immunization.Vaccine. 2010 Nov 10;28(48):7659-66. doi: 10.1016/j.vaccine.2010.09.040. Epub 2010 Sep 25. Vaccine. 2010. PMID: 20875490 Free PMC article.
-
Induction of protection in mice against a respiratory challenge by a vaccine formulated with the Chlamydia major outer membrane protein adjuvanted with IC31®.Vaccine. 2011 Mar 16;29(13):2437-43. doi: 10.1016/j.vaccine.2011.01.031. Epub 2011 Jan 26. Vaccine. 2011. PMID: 21276442
Cited by
-
Chlamydia trachomatis recombinant MOMP encapsulated in PLGA nanoparticles triggers primarily T helper 1 cellular and antibody immune responses in mice: a desirable candidate nanovaccine.Int J Nanomedicine. 2013;8:2085-99. doi: 10.2147/IJN.S44155. Epub 2013 May 30. Int J Nanomedicine. 2013. PMID: 23785233 Free PMC article.
-
A Recombinant Chlamydia trachomatis MOMP Vaccine Elicits Cross-serogroup Protection in Mice Against Vaginal Shedding and Infertility.J Infect Dis. 2020 Jan 2;221(2):191-200. doi: 10.1093/infdis/jiz438. J Infect Dis. 2020. PMID: 31504647 Free PMC article.
-
Pathogenicity and virulence of Chlamydia trachomatis: Insights into host interactions, immune evasion, and intracellular survival.Virulence. 2025 Dec;16(1):2503423. doi: 10.1080/21505594.2025.2503423. Epub 2025 May 15. Virulence. 2025. PMID: 40353442 Free PMC article. Review.
-
Structural Assessment of Chlamydia trachomatis Major Outer Membrane Protein (MOMP)-Derived Vaccine Antigens and Immunological Profiling in Mice with Different Genetic Backgrounds.Vaccines (Basel). 2024 Jul 18;12(7):789. doi: 10.3390/vaccines12070789. Vaccines (Basel). 2024. PMID: 39066427 Free PMC article.
-
Vaccination with the recombinant major outer membrane protein elicits antibodies to the constant domains and induces cross-serovar protection against intranasal challenge with Chlamydia trachomatis.Infect Immun. 2013 May;81(5):1741-50. doi: 10.1128/IAI.00734-12. Epub 2013 Mar 11. Infect Immun. 2013. PMID: 23478318 Free PMC article.
References
-
- Miller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–2236. - PubMed
-
- Schachter J, Dawson C. Human chlamydial infections. Littleton: PSG Publishing Co; 1978.
-
- Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. - PubMed
-
- Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. - PubMed
-
- Dean D, Suchland RJ, Stamm WE. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J Infect Dis. 2000;182:909–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

