Establishment of cancer cell lines from rat hepatocholangiocarcinoma and assessment of the role of granulocyte-colony stimulating factor and hepatocyte growth factor in their growth, motility and survival
- PMID: 19446912
- PMCID: PMC2694236
- DOI: 10.1016/j.jhep.2009.02.022
Establishment of cancer cell lines from rat hepatocholangiocarcinoma and assessment of the role of granulocyte-colony stimulating factor and hepatocyte growth factor in their growth, motility and survival
Abstract
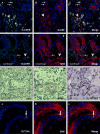
Background/aims: Oval cells (OCs), putative hepatic stem cells, may give rise to liver cancers. We developed a carcinogenesis regimen, based upon induction of OC proliferation prior to carcinogen exposure. In our model, rats subjected to 2-acetylaminofluorene/ partial-hepatectomy followed by aflatoxin injection (APA regimen) developed well-differentiated hepatocholangiocarcinomas. The aim of this study was to establish and characterize cancer cell lines from this animal model.
Methods: Cancer cells were cultured from animals sacrificed eight months after treatment, and single clones were selected. The established cell lines, named LCSCs, were characterized, and their tumorigenicity was assessed in vivo. The roles of granulocyte-colony stimulating factor (G-CSF) and hepatocyte growth factor (HGF) in LCSC growth, survival and motility were also investigated.
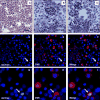
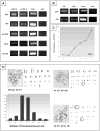
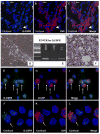
Results: From primary tumors, six cell lines were developed. LCSCs shared with the primary tumors the expression of various OC-associated markers, including cMet and G-CSF receptor. In vitro, HGF conferred protection from death by serum withdrawal. Stimulation with G-CSF increased LCSC growth and motility, while the blockage of its receptor inhibited LCSC proliferation and migration.
Conclusions: Six cancer cell lines were established from our model of hepatocholangiocarcinoma. HGF modulated LCSC resistance to apoptosis, while G-CSF acted on LCSCs as a proliferative and chemotactic agent.
Figures






Similar articles
-
Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats.Gastroenterology. 2007 Aug;133(2):619-31. doi: 10.1053/j.gastro.2007.05.018. Epub 2007 May 21. Gastroenterology. 2007. PMID: 17681181 Free PMC article.
-
In vivo transfer of hepatocyte growth factor gene accelerates proliferation of hepatic oval cells in a 2-acetylaminofluorene/partial hepatectomy model in rats.FEBS Lett. 2000 Mar 31;470(3):325-30. doi: 10.1016/s0014-5793(00)01337-5. FEBS Lett. 2000. PMID: 10745090
-
Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat.Am J Pathol. 1993 Jun;142(6):1823-30. Am J Pathol. 1993. PMID: 8506951 Free PMC article.
-
[HGF/c-Met and metastasis of hepatocellular carcinoma].Zhonghua Gan Zang Bing Za Zhi. 2005 May;13(5):396-8. Zhonghua Gan Zang Bing Za Zhi. 2005. PMID: 15918987 Review. Chinese. No abstract available.
-
Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF): receptor biology, signal transduction, and neutrophil activation.Blood Rev. 1992 Mar;6(1):43-57. doi: 10.1016/0268-960x(92)90007-d. Blood Rev. 1992. PMID: 1375123 Review.
Cited by
-
Characterization of liver injury, oval cell proliferation and cholangiocarcinogenesis in glutathione S-transferase A3 knockout mice.Carcinogenesis. 2017 Jul 1;38(7):717-727. doi: 10.1093/carcin/bgx048. Carcinogenesis. 2017. PMID: 28535182 Free PMC article.
-
Combined Hepatocellular-Cholangiocarcinoma: Biology, Diagnosis, and Management.Liver Cancer. 2023 Apr 24;13(1):6-28. doi: 10.1159/000530700. eCollection 2024 Feb. Liver Cancer. 2023. PMID: 38344449 Free PMC article. Review.
-
Isolation of EpCAM(+)/CD133 (-) hepatic progenitor cells.Mol Cells. 2013 Nov;36(5):424-31. doi: 10.1007/s10059-013-0190-y. Epub 2013 Nov 14. Mol Cells. 2013. PMID: 24293012 Free PMC article.
-
Characterization of an Oncolytic Adenovirus Vector Constructed to Target the cMet Receptor.Oncolytic Virother. 2015;4:119-132. doi: 10.2147/OV.S87369. Oncolytic Virother. 2015. PMID: 26866014 Free PMC article.
-
Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study.Br J Cancer. 2018 Feb 6;118(3):325-330. doi: 10.1038/bjc.2017.413. Epub 2017 Nov 23. Br J Cancer. 2018. PMID: 29169182 Free PMC article.
References
-
- Piscaglia AC, Shupe DT, Petersen BE, Gasbarrini A. Stem Cells, Cancer, Liver, and Liver Cancer Stem Cells: Finding a Way out of the Labyrinth…. Curr Cancer Drug Targets. 2007;7:582–590. - PubMed
-
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. - PubMed
-
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. - PubMed
-
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

