Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial
- PMID: 19447249
- PMCID: PMC2687939
- DOI: 10.1016/S0140-6736(09)60740-6
Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial
Abstract
Background: Incorporation of a taxane as adjuvant treatment for early breast cancer offers potential for further improvement of anthracycline-based treatment. The UK TACT study (CRUK01/001) investigated whether sequential docetaxel after anthracycline chemotherapy would improve patient outcome compared with standard chemotherapy of similar duration.
Methods: In this multicentre, open-label, phase III, randomised controlled trial, 4162 women (aged >18 years) with node-positive or high-risk node-negative operable early breast cancer were randomly assigned by computer-generated permuted block randomisation to receive FEC (fluorouracil 600 mg/m(2), epirubicin 60 mg/m(2), cyclophosphamide 600 mg/m(2) at 3-weekly intervals) for four cycles followed by docetaxel (100 mg/m(2) at 3-weekly intervals) for four cycles (n=2073) or control (n=2089). For the control regimen, centres chose either FEC for eight cycles (n=1265) or epirubicin (100 mg/m(2) at 3-weekly intervals) for four cycles followed by CMF (cyclophosphamide 600 mg/m(2), methotrexate 40 mg/m(2), and fluorouracil 600 mg/m(2) at 4-weekly intervals) for four cycles (n=824). The primary endpoint was disease-free survival. Analysis was by intention to treat (ITT). This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN79718493.
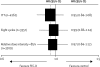
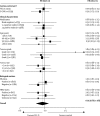
Findings: All randomised patients were included in the ITT population. With a median follow-up of 62 months, disease-free survival events were seen in 517 of 2073 patients in the experimental group compared with 539 of 2089 controls (hazard ratio [HR] 0.95, 95% CI 0.85-1.08; p=0.44). 75.6% (95% CI 73.7-77.5) of patients in the experimental group and 74.3% (72.3-76.2) of controls were alive and disease-free at 5 years. The proportion of patients who reported any acute grade 3 or 4 adverse event was significantly greater in the experimental group than in the control group (p<0.0001); the most frequent events were neutropenia (937 events vs 797 events), leucopenia (507 vs 362), and lethargy (456 vs 272).
Interpretation: This study did not show any overall gain from the addition of docetaxel to standard anthracycline chemotherapy. Exploration of predictive biomarker-defined subgroups might have the potential to better target the use of taxane-based therapy.
Funding: Cancer Research UK (CRUK 01/001), Sanofi-Aventis, Pfizer, and Roche.
Figures





Comment in
-
Unravelling the mystery of the TACT trial.Lancet. 2009 May 16;373(9676):1662-3. doi: 10.1016/S0140-6736(09)60925-9. Lancet. 2009. PMID: 19447241 No abstract available.
-
Have we reached a plateau in adjuvant breast cancer therapy?Lancet. 2009 Oct 24;374(9699):1421. doi: 10.1016/S0140-6736(09)61862-6. Lancet. 2009. PMID: 19854372 No abstract available.
References
-
- Early Breast Cancer Trialists' Collaborative Group Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Henderson IC, Berry DA, Demetri GD. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. - PubMed
-
- Mamounas EP, Bryant J, Lembersky B. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. - PubMed
-
- Dowsett M, Bartlett J, Ellis IO. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol. 2003;199:418–423. - PubMed
Uncited reference
-
- Francis P, Crown J, Di Leo A. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst. 2008;100:121–133. - PubMed
-
- Goldstein LJ, O'Neill A, Sparano JA. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J Clin Oncol. 2008;26:4092–4099. - PMC - PubMed
-
- Jones S, Holmes FA, O'Shaughnessy J. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. - PubMed
-
- Martin M, Pienkowski T, Mackey J. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. - PubMed
-
- Martin M, Lluch A, Segui MA. TAC versus FAC as adjuvant chemotherapy for high-risk node-negative breast cancer: results of the GEICAM 9805 trial. Ann Oncol. 2008;19(suppl 8):viii77–viii88. abstract 1830.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

