Processing of alkylcobalamins in mammalian cells: A role for the MMACHC (cblC) gene product
- PMID: 19447654
- PMCID: PMC2709701
- DOI: 10.1016/j.ymgme.2009.04.005
Processing of alkylcobalamins in mammalian cells: A role for the MMACHC (cblC) gene product
Abstract
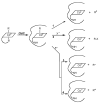
The MMACHC gene product of the cblC complementation group, referred to as the cblC protein, catalyzes the in vitro and in vivo decyanation of cyanocobalamin (vitamin B(12)). We hypothesized that the cblC protein would also catalyze the dealkylation of newly internalized methylcobalamin (MeCbl) and 5'-deoxyadenosylcobalamin (AdoCbl), the naturally occurring alkylcobalamins that are present in the diet. The hypothesis was tested in cultured endothelial cells using [(57)Co]-AdoCbl and MeCbl analogs consisting of [(57)Co]-labeled straight-chain alkylcobalamins ranging from C2 (ethylcobalamin) to C6 (hexylcobalamin). [(57)Co]-AdoCbl was converted to [(57)Co]-MeCbl by cultured bovine aortic endothelial cells, suggesting that a dealkylation process likely involving the cblC protein removed the 5'-deoxyadenosyl alkyl group. Surprisingly, all of the straight-chain alkylcobalamins served as substrates for the biosynthesis of both AdoCbl and MeCbl. Dealkylation was then assessed in normal skin fibroblasts and fibroblasts derived from three patients with mutations in the MMACHC gene. While normal skin fibroblasts readily converted [(57)Co]-propylcobalamin to [(57)Co]-AdoCbl and [(57)Co]-MeCbl, there was little or no conversion in cblC mutant fibroblasts. These studies suggest that the CblC protein is responsible for early processing of both CNCbl (decyanation) and alkylcobalamins (dealkylation) in mammalian cells.
Figures

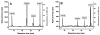

References
-
- Rosenblatt DS, Fenton WA. Inherited disorders of folate and cobalamin transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic & Molecular Bases of Inherited Disease. Mc-Graw Hill; New York: 2001. pp. 3897–3933.
-
- Quadros EV, Jackson B, Hoffbrand AV, et al. Interconversion of cobalamins in human lymphocytes in vitro and the influence of nitrous oxide on sythesis of cobalamin coenzymes. In: Zagalak B, Friedrich W, editors. Vitamin B12. Walter de Gruyter; Berlin: 1979. pp. 1045–1054.
-
- Suormala T, Baumgartner MR, Coelho D, et al. The cblD defect causes either isolated or combined deficiency of methylcobalamin and adenosylcobalamin synthesis. J Biol Chem. 2004;279:42742–42749. - PubMed
-
- Lerner-Ellis JP, Tirone JC, Pawelek PD, et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet. 2006;38:93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

