Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk
- PMID: 19448084
- PMCID: PMC2683015
- DOI: 10.1242/jeb.027284
Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk
Abstract
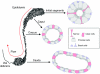
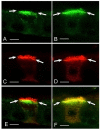
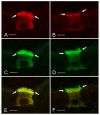
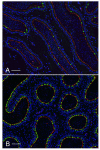
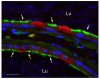
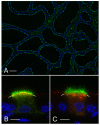
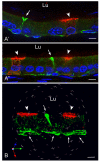
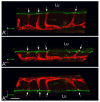
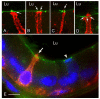
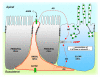
In the epididymis, spermatozoa acquire their ability to become motile and to fertilize an egg. A luminal acidic pH and a low bicarbonate concentration help keep spermatozoa in a quiescent state during their maturation and storage in this organ. Net proton secretion is crucial to maintain the acidity of the luminal fluid in the epididymis. A sub-population of epithelial cells, the clear cells, express high levels of the proton-pumping V-ATPase in their apical membrane and are important contributors to luminal acidification. This review describes selected aspects of V-ATPase regulation in clear cells. The assembly of a particular set of V-ATPase subunit isoforms governs the targeting of the pump to the apical plasma membrane. Regulation of V-ATPase-dependent proton secretion occurs via recycling mechanisms. The bicarbonate-activated adenylyl cyclase is involved in the non-hormonal regulation of V-ATPase recycling, following activation of bicarbonate secretion by principal cells. The V-ATPase is also regulated in a paracrine manner by luminal angiotensin II by activation of the angiotensin II type 2 receptor (AGTR2), which is located in basal cells. Basal cells have the remarkable property of extending long and slender cytoplasmic projections that cross the tight junction barrier to monitor the luminal environment. Clear cells are activated by a nitric oxide signal that originates from basal cells. Thus, a complex interplay between the different cell types present in the epithelium leads to activation of the luminal acidifying capacity of the epididymis, a process that is crucial for sperm maturation and storage.
Figures

References
-
- Acott, T. S. and Carr, D. W. (1984). Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol. Reprod. 30, 926-935. - PubMed
-
- Bagnis, C., Marsolais, M., Biemesderfer, D., Laprade, R. and Breton, S. (2001). Na(+)/H(+)-exchange activity and immunolocalization of NHE3 in rat epididymis. Am. J. Physiol. Renal Physiol. 280, F426-F436. - PubMed
-
- Beaulieu, V., Da Silva, N., Pastor-Soler, N., Brown, C. R., Smith, P. J., Brown, D. and Breton, S. (2005). Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+ATPase (V-ATPase) recycling. J. Biol. Chem. 280, 8452-8463. - PubMed
-
- Beyenbach, K. W. and Wieczorek, H. (2006). The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577-589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

