Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking
- PMID: 19448085
- PMCID: PMC2683016
- DOI: 10.1242/jeb.028803
Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking
Abstract
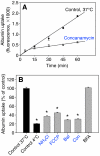
The proton-pumping V-ATPase is a complex, multi-subunit enzyme that is highly expressed in the plasma membranes of some epithelial cells in the kidney, including collecting duct intercalated cells. It is also located on the limiting membranes of intracellular organelles in the degradative and secretory pathways of all cells. Different isoforms of some V-ATPase subunits are involved in the targeting of the proton pump to its various intracellular locations, where it functions in transporting protons out of the cell across the plasma membrane or acidifying intracellular compartments. The former process plays a critical role in proton secretion by the kidney and regulates systemic acid-base status whereas the latter process is central to intracellular vesicle trafficking, membrane recycling and the degradative pathway in cells. We will focus our discussion on two cell types in the kidney: (1) intercalated cells, in which proton secretion is controlled by shuttling V-ATPase complexes back and forth between the plasma membrane and highly-specialized intracellular vesicles, and (2) proximal tubule cells, in which the endocytotic pathway that retrieves proteins from the glomerular ultrafiltrate requires V-ATPase-dependent acidification of post-endocytotic vesicles. The regulation of both of these activities depends upon the ability of cells to monitor the pH and/or bicarbonate content of their extracellular environment and intracellular compartments. Recent information about these pH-sensing mechanisms, which include the role of the V-ATPase itself as a pH sensor and the soluble adenylyl cyclase as a bicarbonate sensor, will be addressed in this review.
Figures




References
-
- Al-Awqati, Q. (1996). Plasticity in epithelial polarity of renal intercalated cells: targeting of the H+ATPase and band 3. Am. J. Physiol. 270, C1571-C1580. - PubMed
-
- Al-Awqati, Q. (2003). Terminal differentiation of intercalated cells: the role of hensin. Annu. Rev. Physiol. 65, 567-583. - PubMed
-
- Al-Awqati, Q., Vijayakumar, S., Hikita, C., Chen, J. and Takito, J. (1998). Phenotypic plasticity in the intercalated cell: the hensin pathway. Am. J. Physiol. 275, F183-F190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

