A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder
- PMID: 19448189
- PMCID: PMC3908470
- DOI: 10.1176/appi.ajp.2009.08111633
A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder
Abstract
Objective: Lithium remains a first-line treatment for bipolar disorder, but the mechanisms by which it prevents the recurrence of mood episodes are not known. The authors utilized data from a genomewide association study to examine associations between single nucleotide polymorphisms (SNPs) and the outcome of lithium treatment in two cohorts of patients with bipolar I disorder or bipolar II disorder.
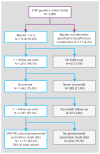
Method: The hazard for mood episode recurrence was examined among 1,177 patients with bipolar I disorder or bipolar II disorder, including 458 individuals treated with lithium carbonate or citrate, who were participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) cohort. SNPs showing the greatest evidence of association in Cox regression models were then examined for association with positive lithium response among 359 bipolar I or II disorder patients treated with lithium carbonate or citrate in a second cohort from the University College London.
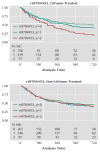
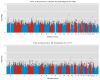
Results: The strongest association in the STEP-BD cohort (minimum p=5.5 x 10(-7)) was identified for a region on chromosome 10p15 (rs10795189). Of the regions showing suggestive evidence (p<5 x 10(-4)) of association with lithium response, five were further associated with positive lithium response in the University College London cohort, including SNPs in a region on chromosome 4q32 spanning a gene coding for the glutamate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate (AMPA) receptor GRIA2.
Conclusions: Multiple novel loci merit further examination for association with lithium response in bipolar disorder patients, including one region that spans the GRIA2 gene, for which expression has been shown to be regulated by lithium treatment.
Figures
Comment in
-
New hope for pharmacogenetic testing.Am J Psychiatry. 2009 Jun;166(6):635-8. doi: 10.1176/appi.ajp.2009.09040536. Am J Psychiatry. 2009. PMID: 19487397 No abstract available.
References
-
- Tohen M, Zarate CA, Jr, Hennen J, Khalsa HM, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. - PubMed
-
- Carlson GA, Bromet EJ, Driessens C, Mojtabai R, Schwartz JE. Age at onset, childhood psychopathology, and 2-year outcome in psychotic bipolar disorder. Am J Psychiatry. 2002;159:307–309. - PubMed
-
- Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. - PubMed
-
- Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161:1537–1547. - PubMed
-
- Hirschfeld RM, Bowden CL, Gitlin MJ, Keck PE, Perlis RH, Suppes T, Thase ME. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159(April suppl):1–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- D43 TW009114/TW/FIC NIH HHS/United States
- MH-062137/MH/NIMH NIH HHS/United States
- R01 MH062137/MH/NIMH NIH HHS/United States
- N01 MH080001/MH/NIMH NIH HHS/United States
- G9623693N/MRC_/Medical Research Council/United Kingdom
- MH-067288/MH/NIMH NIH HHS/United States
- G0500791/MRC_/Medical Research Council/United Kingdom
- MH63420/MH/NIMH NIH HHS/United States
- R01 MH067288/MH/NIMH NIH HHS/United States
- MH-063445/MH/NIMH NIH HHS/United States
- R01 MH063420/MH/NIMH NIH HHS/United States
- R01 MH063445/MH/NIMH NIH HHS/United States

