The cation diffusion facilitator gene cdf-2 mediates zinc metabolism in Caenorhabditis elegans
- PMID: 19448268
- PMCID: PMC2728845
- DOI: 10.1534/genetics.109.103614
The cation diffusion facilitator gene cdf-2 mediates zinc metabolism in Caenorhabditis elegans
Abstract
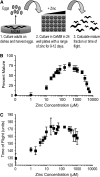
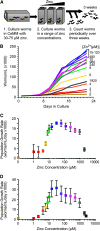
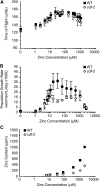
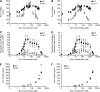
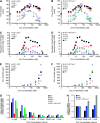
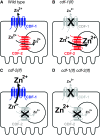
Zinc is essential for many cellular processes. To use Caenorhabditis elegans to study zinc metabolism, we developed culture conditions allowing full control of dietary zinc and methods to measure zinc content of animals. Dietary zinc dramatically affected growth and zinc content; wild-type worms survived from 7 microm to 1.3 mm dietary zinc, and zinc content varied 27-fold. We investigated cdf-2, which encodes a predicted zinc transporter in the cation diffusion facilitator family. cdf-2 mRNA levels were increased by high dietary zinc, suggesting cdf-2 promotes zinc homeostasis. CDF-2 protein was expressed in intestinal cells and localized to cytosolic vesicles. A cdf-2 loss-of-function mutant displayed impaired growth and reduced zinc content, indicating that CDF-2 stores zinc by transport into the lumen of vesicles. The relationships between three cdf genes, cdf-1, cdf-2, and sur-7, were analyzed in double and triple mutant animals. A cdf-1 mutant displayed increased zinc content, whereas a cdf-1 cdf-2 double mutant had intermediate zinc content, suggesting cdf-1 and cdf-2 have antagonistic functions. These studies advance C. elegans as a model of zinc metabolism and identify cdf-2 as a new gene that has a critical role in zinc storage.
Figures


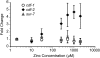

Similar articles
-
Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans.Cell Metab. 2012 Jan 4;15(1):88-99. doi: 10.1016/j.cmet.2011.12.003. Cell Metab. 2012. PMID: 22225878 Free PMC article.
-
ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis.PLoS Genet. 2013 May;9(5):e1003522. doi: 10.1371/journal.pgen.1003522. Epub 2013 May 23. PLoS Genet. 2013. PMID: 23717214 Free PMC article.
-
The role of cation diffusion facilitator CDF-1 in lipid metabolism in Caenorhabditis elegans.G3 (Bethesda). 2021 Jul 14;11(7):jkab120. doi: 10.1093/g3journal/jkab120. G3 (Bethesda). 2021. PMID: 33871589 Free PMC article.
-
A structural overview of the zinc transporters in the cation diffusion facilitator family.Acta Crystallogr D Struct Biol. 2019 Apr 1;75(Pt 4):357-367. doi: 10.1107/S2059798319003814. Epub 2019 Apr 5. Acta Crystallogr D Struct Biol. 2019. PMID: 30988253 Free PMC article. Review.
-
Fine-tuning the RAS signaling pathway: Zn(2+) makes the difference.Mol Cell. 2002 May;9(5):927-8. doi: 10.1016/s1097-2765(02)00535-x. Mol Cell. 2002. PMID: 12049729 Review.
Cited by
-
A modular system of DNA enhancer elements mediates tissue-specific activation of transcription by high dietary zinc in C. elegans.Nucleic Acids Res. 2015 Jan;43(2):803-16. doi: 10.1093/nar/gku1360. Epub 2014 Dec 30. Nucleic Acids Res. 2015. PMID: 25552416 Free PMC article.
-
Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans.Cell Metab. 2012 Jan 4;15(1):88-99. doi: 10.1016/j.cmet.2011.12.003. Cell Metab. 2012. PMID: 22225878 Free PMC article.
-
Zinc Levels Modulate Lifespan through Multiple Longevity Pathways in Caenorhabditis elegans.PLoS One. 2016 Apr 14;11(4):e0153513. doi: 10.1371/journal.pone.0153513. eCollection 2016. PLoS One. 2016. PMID: 27078872 Free PMC article.
-
Cadmium alters whole animal ionome and promotes the re-distribution of iron in intestinal cells of Caenorhabditis elegans.Front Physiol. 2023 Sep 26;14:1258540. doi: 10.3389/fphys.2023.1258540. eCollection 2023. Front Physiol. 2023. PMID: 37822680 Free PMC article.
-
Zinc mediates the SREBP-SCD axis to regulate lipid metabolism in Caenorhabditis elegans.J Lipid Res. 2017 Sep;58(9):1845-1854. doi: 10.1194/jlr.M077198. Epub 2017 Jul 14. J Lipid Res. 2017. PMID: 28710073 Free PMC article.
References
-
- Andrews, G. K., H. Wang, S. K. Dey and R. D. Palmiter, 2004. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis 40 74–81. - PubMed
-
- Beitel, G. J., S. G. Clark and H. R. Horvitz, 1990. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348 503–509. - PubMed
-
- Bruinsma, J. J., T. Jirakulaporn, A. J. Muslin and K. Kornfeld, 2002. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev. Cell 2 567–578. - PubMed
-
- Bruinsma, J. J., D. L. Schneider, D. E. Davis and K. Kornfeld, 2008. Identification of mutations in Caenorhabditis elegans that cause resistance to high levels of dietary zinc and analysis using a genomewide map of single nucleotide polymorphisms scored by pyrosequencing. Genetics 179 811–828. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

