Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys
- PMID: 19448633
- PMCID: PMC2723177
- DOI: 10.1038/nm.1967
Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys
Abstract
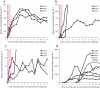
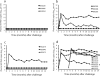
The key to an effective HIV vaccine is development of an immunogen that elicits persisting antibodies with broad neutralizing activity against field strains of the virus. Unfortunately, very little progress has been made in finding or designing such immunogens. Using the simian immunodeficiency virus (SIV) model, we have taken a markedly different approach: delivery to muscle of an adeno-associated virus gene transfer vector expressing antibodies or antibody-like immunoadhesins having predetermined SIV specificity. With this approach, SIV-specific molecules are endogenously synthesized in myofibers and passively distributed to the circulatory system. Using such an approach in monkeys, we have now generated long-lasting neutralizing activity in serum and have observed complete protection against intravenous challenge with virulent SIV. In essence, this strategy bypasses the adaptive immune system and holds considerable promise as a unique approach to an effective HIV vaccine.
Figures

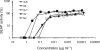
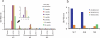
Comment in
-
Blocking and tackling HIV.Nat Med. 2009 Aug;15(8):841-2. doi: 10.1038/nm0809-841. Nat Med. 2009. PMID: 19661984 No abstract available.
References
-
- Flynn NM, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. - PubMed
-
- Pitisuttithum P, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. - PubMed
-
- Desrosiers R. Prospects for an AIDS vaccine. Nat Med. 2004;10:221–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

