Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle
- PMID: 19448704
- PMCID: PMC2748314
- DOI: 10.1139/H09-044
Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle
Abstract
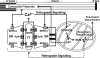
The skeletal muscle contractile machine is fueled by both calcium and ATP. Calcium ions activate the contractile machinery by binding to troponin C and relieving troponin-tropomyosin inhibition of actinomyosin interaction. ATP binding to myosin during the contractile cycle results in myosin detachment from actin, and energy liberated from subsequent ATP hydrolysis is then used to drive the next contractile cycle. ATP is also used to lower myoplasmic calcium levels during muscle relaxation. Thus, muscle contractility is intimately linked to the proper control of sarcomeric Ca2+ delivery and (or) removal and ATP generation and (or) utilization. In skeletal muscle, the sarcoplasmic reticulum (SR) is the primary regulator of calcium storage, release, and reuptake, while glycolysis and the mitochondria are responsible for cellular ATP production. However, the SR and mitochondrial function in muscle are not independent, as calcium uptake into the mitochondria increases ATP production by stimulating oxidative phosphorylation and mitochondrial ATP production, and production and (or) detoxification of reactive oxygen and nitrogen species (ROS/RNS), in turn, modulates SR calcium release and reuptake. Close spatial Ca2+/ATP/ROS/RNS communication between the SR and mitochondria is facilitated by the structural attachment of mitochondria to the calcium release unit (CRU) by 10 nm of electron-dense tethers. The resultant anchoring of mitochondria to the CRU provides a structural basis for maintaining bidirectional SR-mitochondrial through-space communication during vigorous contraction. This review will consider the degree to which this structural link enables privileged or microdomain communication between the SR and mitochondria in skeletal muscle.
Figures


References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 2008;88:287–332. - PubMed
-
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 2002;34:1259–1271. - PubMed
-
- Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001;276:21482–21488. - PubMed
-
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

