Changing nuclear landscape and unique PML structures during early epigenetic transitions of human embryonic stem cells
- PMID: 19449340
- PMCID: PMC2937361
- DOI: 10.1002/jcb.22183
Changing nuclear landscape and unique PML structures during early epigenetic transitions of human embryonic stem cells
Abstract
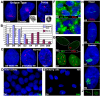
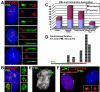
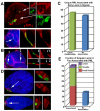
The complex nuclear structure of somatic cells is important to epigenomic regulation, yet little is known about nuclear organization of human embryonic stem cells (hESC). Here we surveyed several nuclear structures in pluripotent and transitioning hESC. Observations of centromeres, telomeres, SC35 speckles, Cajal Bodies, lamin A/C and emerin, nuclear shape and size demonstrate a very different "nuclear landscape" in hESC. This landscape is remodeled during a brief transitional window, concomitant with or just prior to differentiation onset. Notably, hESC initially contain abundant signal for spliceosome assembly factor, SC35, but lack discrete SC35 domains; these form as cells begin to specialize, likely reflecting cell-type specific genomic organization. Concomitantly, nuclear size increases and shape changes as lamin A/C and emerin incorporate into the lamina. During this brief window, hESC exhibit dramatically different PML-defined structures, which in somatic cells are linked to gene regulation and cancer. Unlike the numerous, spherical somatic PML bodies, hES cells often display approximately 1-3 large PML structures of two morphological types: long linear "rods" or elaborate "rosettes", which lack substantial SUMO-1, Daxx, and Sp100. These occur primarily between Day 0-2 of differentiation and become rare thereafter. PML rods may be "taut" between other structures, such as centromeres, but clearly show some relationship with the lamina, where PML often abuts or fills a "gap" in early lamin A/C staining. Findings demonstrate that pluripotent hES cells have a markedly different overall nuclear architecture, remodeling of which is linked to early epigenomic programming and involves formation of unique PML-defined structures.
2009 Wiley-Liss, Inc.
Figures



References
-
- Bartova E, Galiova G, Krejci J, Harnicarova A, Strasak L, Kozubek S. Epigenome and chromatin structure in human embryonic stem cells undergoing differentiation. Dev Dyn. 2008a;237:3690–702. - PubMed
-
- Bartova E, Krejci J, Harnicarova A, Kozubek S. Differentiation of human embryonic stem cells induces condensation of chromosome territories and formation of heterochromatin protein 1 foci. Differentiation. 2008b;76:24–32. - PubMed
-
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–93. - PubMed
-
- Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

