TAT-mediated intracellular protein delivery to primary brain cells is dependent on glycosaminoglycan expression
- PMID: 19449355
- PMCID: PMC2729539
- DOI: 10.1002/bit.22377
TAT-mediated intracellular protein delivery to primary brain cells is dependent on glycosaminoglycan expression
Abstract
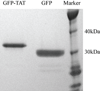
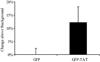
Although some studies have shown that the cell penetrating peptide (CPP) TAT can enter a variety of cell lines with high efficiency, others have observed little or no transduction in vivo or in vitro under conditions mimicking the in vivo environment. The mechanisms underlying TAT-mediated transduction have been investigated in cell lines, but not in primary brain cells. In this study we demonstrate that transduction of a green fluorescent protein (GFP)-TAT fusion protein is dependent on glycosaminoglycan (GAG) expression in both the PC12 cell line and primary astrocytes. GFP-TAT transduced PC12 cells and did so with even higher efficiency following NGF differentiation. In cultures of primary brain cells, TAT significantly enhanced GFP delivery into astrocytes grown under different conditions: (1) monocultures grown in serum-containing medium; (2) monocultures grown in serum-free medium; (3) cocultures with neurons in serum-free medium. The efficiency of GFP-TAT transduction was significantly higher in the monocultures than in the cocultures. The GFP-TAT construct did not significantly enter neurons. Experimental modulation of GAG content correlated with alterations in TAT transduction in PC12 cells and astrocyte monocultures grown in the presence of serum. In addition, this correlation was predictive of TAT-mediated transduction in astrocyte monocultures grown in serum free medium and in coculture. We conclude that culture conditions affect cellular GAG expression, which in turn dictates TAT-mediated transduction efficiency, extending previous results from cell lines to primary cells. These results highlight the cell-type and phenotype-dependence of TAT-mediated transduction, and underscore the necessity of controlling the phenotype of the target cell in future protein engineering efforts aimed at creating more efficacious CPPs.
(c) 2009 Wiley Periodicals, Inc.
Figures




Similar articles
-
Attenuation of astrocyte activation by TAT-mediated delivery of a peptide JNK inhibitor.J Neurotrauma. 2011 Jul;28(7):1219-28. doi: 10.1089/neu.2011.1879. J Neurotrauma. 2011. PMID: 21510821
-
Increased delivery of TAT across an endothelial monolayer following ischemic injury.Neurosci Lett. 2010 Dec 3;486(1):1-4. doi: 10.1016/j.neulet.2010.09.029. Epub 2010 Sep 17. Neurosci Lett. 2010. PMID: 20851169 Free PMC article.
-
A novel use of TAT-EGFP to validate techniques to alter osteosarcoma cell surface glycosaminoglycan expression.J Mol Histol. 2007 Oct;38(5):435-47. doi: 10.1007/s10735-007-9136-z. Epub 2007 Sep 21. J Mol Histol. 2007. PMID: 17885814
-
Delivery of therapeutic proteins as secretable TAT fusion products.Mol Ther. 2009 Feb;17(2):334-42. doi: 10.1038/mt.2008.256. Epub 2008 Dec 2. Mol Ther. 2009. PMID: 19050698 Free PMC article.
-
Doxycycline-inducible and astrocyte-specific HIV-1 Tat transgenic mice (iTat) as an HIV/neuroAIDS model.J Neurovirol. 2018 Apr;24(2):168-179. doi: 10.1007/s13365-017-0598-9. Epub 2017 Nov 15. J Neurovirol. 2018. PMID: 29143286 Free PMC article. Review.
Cited by
-
A bifurcated proteoglycan binding small molecule carrier for siRNA delivery.Chem Biol Drug Des. 2014 Jul;84(1):24-35. doi: 10.1111/cbdd.12295. Epub 2014 May 13. Chem Biol Drug Des. 2014. PMID: 24472581 Free PMC article.
-
An unusual cell penetrating peptide identified using a plasmid display-based functional selection platform.ACS Chem Biol. 2011 May 20;6(5):484-91. doi: 10.1021/cb100423u. Epub 2011 Feb 22. ACS Chem Biol. 2011. PMID: 21291271 Free PMC article.
-
The Cysteine-Containing Cell-Penetrating Peptide AP Enables Efficient Macromolecule Delivery to T Cells and Controls Autoimmune Encephalomyelitis.Pharmaceutics. 2021 Jul 25;13(8):1134. doi: 10.3390/pharmaceutics13081134. Pharmaceutics. 2021. PMID: 34452095 Free PMC article.
-
Brain-targeted drug delivery - nanovesicles directed to specific brain cells by brain-targeting ligands.J Nanobiotechnology. 2024 May 17;22(1):260. doi: 10.1186/s12951-024-02511-7. J Nanobiotechnology. 2024. PMID: 38760847 Free PMC article. Review.
-
SialoPen peptides are new cationic foldamers with remarkable cell permeability.Heliyon. 2020 Dec 24;6(12):e05780. doi: 10.1016/j.heliyon.2020.e05780. eCollection 2020 Dec. Heliyon. 2020. PMID: 33409387 Free PMC article.
References
-
- Audouy S, Mallet J, Privat A, Gimenez y, Ribotta M. Adenovirus-mediated suicide gene therapy in an in vitro model of reactive gliosis. Glia. 1999;25(3):293–303. - PubMed
-
- Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45. - PubMed
-
- Cai SR, Xu G, Becker-Hapak M, Ma M, Dowdy SF, McLeod HL. The kinetics and tissue distribution of protein transduction in mice. Eur J Pharm Sci. 2006;27(4):311–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

