Intracardiac injection of erythropoietin induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model
- PMID: 19449462
- PMCID: PMC3822874
- DOI: 10.1111/j.1582-4934.2008.00546.x
Intracardiac injection of erythropoietin induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model
Abstract
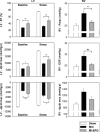
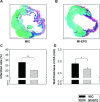
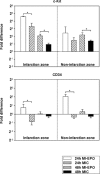
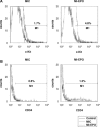
Erythropoietin (EPO) protects the myocardium from ischaemic injury and promotes beneficial remodelling. We assessed the therapeutic efficacy of intracardiac EPO injection and EPO-mediated stem cell homing in a rat myocardial infarction (MI) model. Following MI, EPO (3000 U/kg) or saline was delivered by intracardiac injection. Compared to myocardial infarction control group (MIC), EPO significantly improved left ventricular function (n =11-14, P < 0.05) and decreased right ventricular wall stress (n = 8, P < 0.05) assessed by pressure-volume loops after 6 weeks. MI-EPO hearts exhibited smaller infarction size (20.1 +/- 1.1% versus 27.8 +/- 1.2%; n = 6-8, P < 0.001) and greater capillary density (338.5 +/- 14.7 versus 259.8 +/- 9.2 vessels per mm2; n = 6-8, P < 0.001) than MIC hearts. Direct EPO injection reduced post-MI myocardial apoptosis by approximately 41% (0.27 +/- 0.03% versus 0.42 +/- 0.03%; n = 6, P= 0.005). The chemoattractant SDF-1 was up-regulated significantly assessed by quantitative realtime PCR and immunohistology. c-Kit(+) and CD34(+) stem cells were significantly more numerous in MI-EPO than in MIC at 24 hrs in peripheral blood (n = 7, P < 0.05) and 48 hrs in the infarcted hearts (n = 6, P < 0.001). Further, the mRNAs of Akt, eNOS and EPO receptor were significantly enhanced in MI-EPO hearts (n = 7, P < 0.05). Intracardiac EPO injection restores myocardial functions following MI, which may attribute to the improved early recruitment of c-Kit(+) and CD34(+) stem cells via the enhanced expression of chemoattractant SDF-1.
Figures













Similar articles
-
Intracardiac injection of matrigel induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model.J Cell Mol Med. 2011 Jun;15(6):1310-8. doi: 10.1111/j.1582-4934.2010.01086.x. Epub 2010 May 14. J Cell Mol Med. 2011. PMID: 20477905 Free PMC article.
-
Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis.FASEB J. 2009 Feb;23(2):351-61. doi: 10.1096/fj.08-109462. Epub 2008 Sep 30. FASEB J. 2009. PMID: 18827024
-
Single high-dose intramyocardial administration of erythropoietin promotes early intracardiac proliferation, proves safety and restores cardiac performance after myocardial infarction in rats.Interact Cardiovasc Thorac Surg. 2009 Jul;9(1):20-5; discussion 25. doi: 10.1510/icvts.2008.191916. Epub 2009 Apr 20. Interact Cardiovasc Thorac Surg. 2009. PMID: 19380336
-
SDF-1α as a therapeutic stem cell homing factor in myocardial infarction.Pharmacol Ther. 2011 Jan;129(1):97-108. doi: 10.1016/j.pharmthera.2010.09.011. Epub 2010 Oct 20. Pharmacol Ther. 2011. PMID: 20965212 Review.
-
Erythropoietin: new horizon in cardiovascular medicine.Recent Pat Cardiovasc Drug Discov. 2007 Jan;2(1):5-12. doi: 10.2174/157489007779606112. Recent Pat Cardiovasc Drug Discov. 2007. PMID: 18221097 Review.
Cited by
-
Cardioprotective effects of erythropoietin in diabetic rats determined by CD34 and vascular endothelial growth factor levels.Arch Med Sci Atheroscler Dis. 2020 Jan 19;5:e1-e12. doi: 10.5114/amsad.2020.92346. eCollection 2020. Arch Med Sci Atheroscler Dis. 2020. PMID: 33585719 Free PMC article.
-
Localized SDF-1alpha gene release mediated by collagen substrate induces CD117 stem cells homing.J Cell Mol Med. 2010 Jan;14(1-2):392-402. doi: 10.1111/j.1582-4934.2008.00624.x. Epub 2008 Dec 24. J Cell Mol Med. 2010. PMID: 19413887 Free PMC article.
-
Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction.Circ Res. 2010 Sep 3;107(5):667-76. doi: 10.1161/CIRCRESAHA.110.223289. Epub 2010 Jul 15. Circ Res. 2010. PMID: 20634485 Free PMC article.
-
Erythropoietin attenuates the sequels of ischaemic spinal cord injury with enhanced recruitment of CD34+ cells in mice.J Cell Mol Med. 2012 Aug;16(8):1792-802. doi: 10.1111/j.1582-4934.2011.01489.x. J Cell Mol Med. 2012. PMID: 22145921 Free PMC article.
-
Cell-based therapy for prevention and reversal of myocardial remodeling.Am J Physiol Heart Circ Physiol. 2012 Aug 1;303(3):H256-70. doi: 10.1152/ajpheart.00221.2012. Epub 2012 May 25. Am J Physiol Heart Circ Physiol. 2012. PMID: 22636682 Free PMC article. Review.
References
-
- Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673–86. - PubMed
-
- Zwezdaryk KJ, Coffelt SB, Figueroa YG, Liu J, Phinney DG, LaMarca HL, Florez L, Morris CB, Hoyle GW, Scandurro AB. Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol. 2007;35:640–52. - PubMed
-
- Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-depend-ent pathway. Biochem Biophys Res Commun. 2003;308:990–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

