Carborane clusters in computational drug design: a comparative docking evaluation using AutoDock, FlexX, Glide, and Surflex
- PMID: 19449853
- PMCID: PMC2702476
- DOI: 10.1021/ci900031y
Carborane clusters in computational drug design: a comparative docking evaluation using AutoDock, FlexX, Glide, and Surflex
Abstract
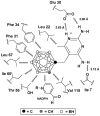
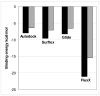
Compounds containing boron atoms play increasingly important roles in the therapy and diagnosis of various diseases, particularly cancer. However, computational drug design of boron-containing therapeutics and diagnostics is hampered by the fact that many software packages used for this purpose lack parameters for all or part of the various types of boron atoms. In the present paper, we describe simple and efficient strategies to overcome this problem, which are based on the replacement of boron atom types with carbon atom types. The developed methods were validated by docking closo- and nido-carboranyl antifolates into the active site of a human dihydrofolate reductase (hDHFR) using AutoDock, Glide, FlexX, and Surflex and comparing the obtained docking poses with the poses of their counterparts in the original hDHFR-carboranyl antifolate crystal structures. Under optimized conditions, AutoDock and Glide were equally good in docking of the closo-carboranyl antifolates followed by Surflex and FlexX, whereas Autodock, Glide, and Surflex proved to be comparably efficient in the docking of nido-carboranyl antifolates followed by FlexX. Differences in geometries and partial atom charges in the structures of the carboranyl antifolates resulting from different data sources and/or optimization methods did not impact the docking performances of AutoDock or Glide significantly. Binding energies predicted by all four programs were in accordance with experimental data.
Figures



Similar articles
-
In Silico Study Identified Methotrexate Analog as Potential Inhibitor of Drug Resistant Human Dihydrofolate Reductase for Cancer Therapeutics.Molecules. 2020 Jul 31;25(15):3510. doi: 10.3390/molecules25153510. Molecules. 2020. PMID: 32752079 Free PMC article.
-
Structural analysis of dihydrofolate reductases enables rationalization of antifolate binding affinities and suggests repurposing possibilities.FEBS J. 2016 Mar;283(6):1139-67. doi: 10.1111/febs.13662. Epub 2016 Feb 25. FEBS J. 2016. PMID: 26797763
-
Benchmark of four popular virtual screening programs: construction of the active/decoy dataset remains a major determinant of measured performance.J Cheminform. 2016 Oct 17;8:56. doi: 10.1186/s13321-016-0167-x. eCollection 2016. J Cheminform. 2016. PMID: 27803745 Free PMC article.
-
Molecular docking towards drug discovery.J Mol Recognit. 1996 Mar-Apr;9(2):175-86. doi: 10.1002/(sici)1099-1352(199603)9:2<175::aid-jmr260>3.0.co;2-d. J Mol Recognit. 1996. PMID: 8877811 Review.
-
Challenges and Opportunities for the Application of Boron Clusters in Drug Design.J Med Chem. 2016 Sep 8;59(17):7738-58. doi: 10.1021/acs.jmedchem.5b01932. Epub 2016 May 10. J Med Chem. 2016. PMID: 27124656 Review.
Cited by
-
Synthesis, biological evaluation, and radioiodination of halogenated closo-carboranylthymidine analogues.Inorg Chem. 2012 Jan 2;51(1):629-39. doi: 10.1021/ic202150b. Epub 2011 Dec 16. Inorg Chem. 2012. PMID: 22175713 Free PMC article.
-
Carboranes in drug discovery, chemical biology and molecular imaging.Nat Rev Chem. 2022 Jul;6(7):486-504. doi: 10.1038/s41570-022-00400-x. Epub 2022 Jun 23. Nat Rev Chem. 2022. PMID: 37117309 Review.
-
Discovery of Novel Nonpeptidic Proteasome Inhibitors Using Covalent Virtual Screening and Biological Evaluation.ACS Med Chem Lett. 2024 Sep 9;15(10):1741-1748. doi: 10.1021/acsmedchemlett.4c00321. eCollection 2024 Oct 10. ACS Med Chem Lett. 2024. PMID: 39411540
-
Docking studies and network analyses reveal capacity of compounds from Kandelia rheedii to strengthen cellular immunity by interacting with host proteins during tuberculosis infection.Bioinformation. 2012;8(21):1012-20. doi: 10.6026/97320630081012. Epub 2012 Oct 31. Bioinformation. 2012. PMID: 23275699 Free PMC article.
-
Effect of 1-Ethyl-3-methylimidazolium Tetrafluoroborate and Acetate Ionic Liquids on Stability and Amyloid Aggregation of Lysozyme.Int J Mol Sci. 2022 Jan 11;23(2):783. doi: 10.3390/ijms23020783. Int J Mol Sci. 2022. PMID: 35054967 Free PMC article.
References
-
- Armstrong AF, Valliant JF. The bioinorganic and medicinal chemistry of carboranes: from new drug discovery to molecular imaging and therapy. Dalton Trans. 2007;38:4240–4251. - PubMed
-
- Lesnikowski ZJ. Boron units as pharmacophores - new applications and opportunities of boron cluster chemistry. Collect Czech Chem Commun. 2007;72:1646–1658.
-
- Endo Y, Iijima T, Yaguchi K, Kawachi E, Inoue N, Kagechika H, Kubo A, Itai A. Structure-Activity study of retinoid agonists bearing substituted dicarba-closo-dodecaborane. Relation between retinoidal activity and conformation of two aromatic nuclei. Bioorg Med Chem Lett. 2001;11:1307–1311. - PubMed
-
- Ogawa T, Ohta K, Yoshimi T, Yamazaki H, Suzuki T, Ohta S, Endo Y. m-Carborane bisphenol structure as a pharmacophore for selective estrogen receptor modulators. Bioorg Med Chem Lett. 2006;16:3943–3946. - PubMed
-
- Goto T, Ohta K, Suzuki T, Ohta S, Endo Y. Design and synthesis of novel androgen receptor antagonists with sterically bulky icosahedral carboranes. Bioorg Med Chem. 2005;13:6414–6424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

