Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena
- PMID: 19449957
- PMCID: PMC2842192
- DOI: 10.1115/1.3118773
Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena
Abstract
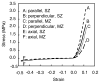
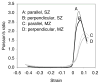
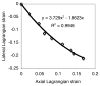
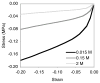
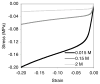
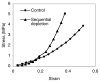
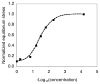
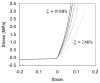
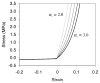
Cartilage is a hydrated soft tissue whose solid matrix consists of negatively charged proteoglycans enmeshed within a fibrillar collagen network. Though many aspects of cartilage mechanics are well understood today, most notably in the context of porous media mechanics, there remain a number of responses observed experimentally whose prediction from theory has been challenging. In this study the solid matrix of cartilage is modeled with a continuous fiber angular distribution, where fibers can only sustain tension, swelled by the osmotic pressure of a proteoglycan ground matrix. It is shown that this representation of cartilage can predict a number of observed phenomena in relation to the tissue's equilibrium response to mechanical and osmotic loading, when flow-dependent and flow-independent viscoelastic effects have subsided. In particular, this model can predict the transition of Poisson's ratio from very low values in compression (approximately 0.02) to very high values in tension (approximately 2.0). Most of these phenomena cannot be explained when using only three orthogonal fiber bundles to describe the tissue matrix, a common modeling assumption used to date. The main picture emerging from this analysis is that the anisotropy of the fibrillar matrix of articular cartilage is intimately dependent on the mechanism of tensed fiber recruitment, in the manner suggested by our recent theoretical study (Ateshian, 2007, ASME J. Biomech. Eng., 129(2), pp. 240-249).
Figures








References
-
- Kempson GE, Freeman MA, Swanson SA. Tensile Properties of Articular Cartilage. Nature. 1968;220(5172):1127–8. - PubMed
-
- Cohen B, Lai WM, Mow VC. A Transversely Isotropic Biphasic Model for Unconfined Compression of Growth Plate and Chondroepiphysis. J Biomech Eng. 1998;120(4):491–6. - PubMed
-
- Loret B, Simoes FMF. Mechanical Effects of Ionic Replacements in Articular Cartilage. Part I: The Constitutive Model. Biomech Model Mechanobiol. 2005;4(2–3):63–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

