Parallel ecological networks in ecosystems
- PMID: 19451126
- PMCID: PMC2685422
- DOI: 10.1098/rstb.2008.0222
Parallel ecological networks in ecosystems
Abstract
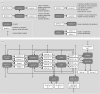
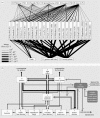
In ecosystems, species interact with other species directly and through abiotic factors in multiple ways, often forming complex networks of various types of ecological interaction. Out of this suite of interactions, predator-prey interactions have received most attention. The resulting food webs, however, will always operate simultaneously with networks based on other types of ecological interaction, such as through the activities of ecosystem engineers or mutualistic interactions. Little is known about how to classify, organize and quantify these other ecological networks and their mutual interplay. The aim of this paper is to provide new and testable ideas on how to understand and model ecosystems in which many different types of ecological interaction operate simultaneously. We approach this problem by first identifying six main types of interaction that operate within ecosystems, of which food web interactions are one. Then, we propose that food webs are structured among two main axes of organization: a vertical (classic) axis representing trophic position and a new horizontal 'ecological stoichiometry' axis representing decreasing palatability of plant parts and detritus for herbivores and detrivores and slower turnover times. The usefulness of these new ideas is then explored with three very different ecosystems as test cases: temperate intertidal mudflats; temperate short grass prairie; and tropical savannah.
Figures






Similar articles
-
Trophic cascades within and across ecosystems: The role of anti-predatory defences, predator type and detritus quality.J Anim Ecol. 2024 Jun;93(6):755-768. doi: 10.1111/1365-2656.14063. Epub 2024 Feb 25. J Anim Ecol. 2024. PMID: 38404168
-
What Can Interaction Webs Tell Us About Species Roles?PLoS Comput Biol. 2015 Jul 21;11(7):e1004330. doi: 10.1371/journal.pcbi.1004330. eCollection 2015 Jul. PLoS Comput Biol. 2015. PMID: 26197151 Free PMC article.
-
Predator effects on a detritus-based food web are primarily mediated by non-trophic interactions.J Anim Ecol. 2014 Jul;83(4):953-62. doi: 10.1111/1365-2656.12189. Epub 2014 Jan 29. J Anim Ecol. 2014. PMID: 24286440
-
The environment to the rescue: can physics help predict predator-prey interactions?Biol Rev Camb Philos Soc. 2024 Dec;99(6):1927-1947. doi: 10.1111/brv.13105. Epub 2024 Jun 10. Biol Rev Camb Philos Soc. 2024. PMID: 38855988 Review.
-
Functional responses and scaling in predator-prey interactions of marine fishes: contemporary issues and emerging concepts.Ecol Lett. 2011 Dec;14(12):1288-99. doi: 10.1111/j.1461-0248.2011.01696.x. Epub 2011 Oct 11. Ecol Lett. 2011. PMID: 21985428 Review.
Cited by
-
Soil networks become more connected and take up more carbon as nature restoration progresses.Nat Commun. 2017 Feb 8;8:14349. doi: 10.1038/ncomms14349. Nat Commun. 2017. PMID: 28176768 Free PMC article.
-
Ecosystem engineering strengthens bottom-up and weakens top-down effects via trait-mediated indirect interactions.Proc Biol Sci. 2017 Sep 27;284(1863):20170894. doi: 10.1098/rspb.2017.0894. Proc Biol Sci. 2017. PMID: 28931733 Free PMC article.
-
Grand challenges in migration biology.Integr Comp Biol. 2010 Sep;50(3):261-79. doi: 10.1093/icb/icq013. Epub 2010 Apr 21. Integr Comp Biol. 2010. PMID: 21558203 Free PMC article.
-
Foundation species enhance food web complexity through non-trophic facilitation.PLoS One. 2018 Aug 31;13(8):e0199152. doi: 10.1371/journal.pone.0199152. eCollection 2018. PLoS One. 2018. PMID: 30169517 Free PMC article.
-
Multivariate Meta-Analysis of Brain-Mass Correlations in Eutherian Mammals.Front Neuroanat. 2016 Sep 30;10:91. doi: 10.3389/fnana.2016.00091. eCollection 2016. Front Neuroanat. 2016. PMID: 27746724 Free PMC article.
References
-
- Aerts R. The freezer defrosting: global warming and litter decomposition rates in cold biomes. J. Ecol. 2006;94:713–724. doi:10.1111/j.1365-2745.2006.01142.x - DOI
-
- Allen T.F.H., Starr T.B. Chicago University Press; Chicago, IL: 1982. Hierarchy.
-
- Allesina S., Alonso D., Pascual M. A general model for food web structure. Science. 2008;320:658–661. doi:10.1126/science.1156269 - DOI - PubMed
-
- Alonso D., Etienne R.S., McKane A.J. The merits of neutral theory. Trends Ecol. Evol. 2006;21:451–457. doi:10.1016/j.tree.2006.03.019 - DOI - PubMed
-
- Andersson J.H., Wijsman J.W.M., Herman P.M.J., Middelburg J.J., Soetaert K., Heip C. Respiration patterns in the deep ocean. Geophys. Res. Lett. 2004;31:L03304. doi:10.1029/2003GL018756 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

