Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans
- PMID: 19451235
- PMCID: PMC2715683
- DOI: 10.1128/IAI.00334-09
Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans
Abstract
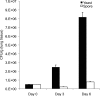
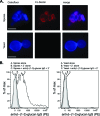
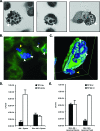
Cryptococcus neoformans was first described as a human fungal pathogen more than a century ago. One aspect of the C. neoformans infectious life cycle that has been the subject of earnest debate is whether the spores are pathogenic. Despite much speculation, no direct evidence has been presented to resolve this outstanding question. We present evidence that C. neoformans spores are pathogenic in a mouse intranasal inhalation model of infection. In addition, we provide mechanistic insights into spore-host interactions. We found that C. neoformans spores were phagocytosed by alveolar macrophages via interactions between fungal beta-(1,3)-glucan and the host receptors Dectin-1 and CD11b. Moreover, we discovered an important link between spore survival and macrophage activation state: intracellular spores were susceptible to reactive oxygen-nitrogen species. We anticipate these results will serve as the basis for a model to further investigate the pathogenic implications of infections caused by fungal spores.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

