Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1
- PMID: 19451266
- PMCID: PMC2715058
- DOI: 10.1084/jem.20082173
Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1
Abstract
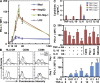
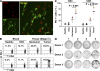
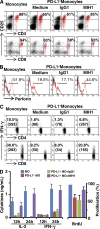
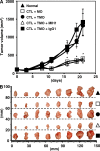
Macrophages (Mphi) are prominent components of solid tumors and exhibit distinct phenotypes in different microenvironments. We have recently found that tumors can alter the normal developmental process of Mphi to trigger transient activation of monocytes in peritumoral stroma. We showed that a fraction of monocytes/Mphi in peritumoral stroma, but not in cancer nests, expresses surface PD-L1 (also termed B7-H1) molecules in tumors from patients with hepatocellular carcinoma (HCC). Monocytes activated by tumors strongly express PD-L1 proteins with kinetics similar to their activation status, and significant correlations were found between the levels of PD-L1(+) and HLA-DR(high) on tumor-infiltrating monocytes. Autocrine tumor necrosis factor alpha and interleukin 10 released from activated monocytes stimulated monocyte expression of PD-L1. The PD-L1(+) monocytes effectively suppressed tumor-specific T cell immunity and contributed to the growth of human tumors in vivo; the effect could be reversed by blocking PD-L1 on those monocytes. Moreover, we found that PD-L1 expression on tumor-infiltrating monocytes increased with disease progression, and the intensity of the protein was associated with high mortality and reduced survival in the HCC patients. Thus, expression of PD-L1 on activated monocytes/Mphi may represent a novel mechanism that links the proinflammatory response to immune tolerance in the tumor milieu.
Figures



References
-
- Mueller M.M., Fusenig N.E. 2004. Friends or foes - bipolar effects of the tumor stroma in cancer.Nat. Rev. Cancer. 4:839–849 - PubMed
-
- Tlsty T.D., Coussens L.M. 2006. Tumor stroma and regulation of cancer development.Annu. Rev. Pathol. 1:119–150 - PubMed
-
- Ahmed F., Steele J.C., Herbert J.M., Steven N.M., Bicknell R. 2008. Tumor stroma as a target in cancer.Curr. Cancer Drug Targets. 8:447–453 - PubMed
-
- Zou W., Chen L. 2008. Inhibitory B7-family molecules in the tumor microenvironment.Nat. Rev. Immunol. 8:467–477 - PubMed
-
- Budhu A., Forgues M., Ye Q.H., Jia H.L., He P., Zanetti K.A., Kammula U.S., Chen Y., Qin L.X., Tang Z.Y., Wang X.W. 2006. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment.Cancer Cell. 10:99–111 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

