TIP47 functions in the biogenesis of lipid droplets
- PMID: 19451273
- PMCID: PMC2711566
- DOI: 10.1083/jcb.200812042
TIP47 functions in the biogenesis of lipid droplets
Abstract
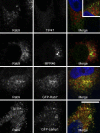
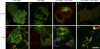
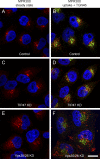
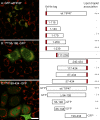
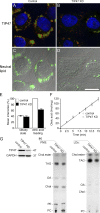
TIP47 (tail-interacting protein of 47 kD) was characterized as a cargo selection device for mannose 6-phosphate receptors (MPRs), directing their transport from endosomes to the trans-Golgi network. In contrast, our current analysis shows that cytosolic TIP47 is not recruited to organelles of the biosynthetic and endocytic pathways. Knockdown of TIP47 expression had no effect on MPR distribution or trafficking and did not affect lysosomal enzyme sorting. Therefore, our data argue against a function of TIP47 as a sorting device. Instead, TIP47 is recruited to lipid droplets (LDs) by an amino-terminal sequence comprising 11-mer repeats. We show that TIP47 has apolipoprotein-like properties and reorganizes liposomes into small lipid discs. Suppression of TIP47 blocked LD maturation and decreased the incorporation of triacylglycerol into LDs. We conclude that TIP47 functions in the biogenesis of LDs.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

