Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect
- PMID: 19451288
- PMCID: PMC2715589
- DOI: 10.1128/AAC.01601-08
Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect
Abstract
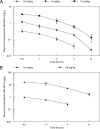
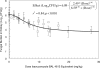
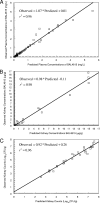
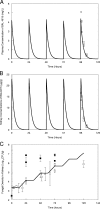
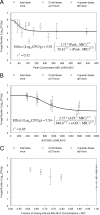
Isavuconazole is a triazole with broad-spectrum activity against medically important fungal pathogens. We investigated the pharmacokinetics and pharmacodynamics of isavuconazole in a murine model of disseminated candidiasis. We determined the pharmacokinetics in both plasma and kidney. The relationship between tissue concentrations and the resultant antifungal effect was described using a mathematical model. The pharmacodynamic parameter that optimally links drug exposure with the antifungal effect was determined using dose fractionation studies. The impact of the immune status of mice receiving isavuconazole was determined in persistently and temporarily neutropenic animals. The pharmacokinetics of 1.6 to 28 mg isavuconazole/kg of body weight were linear. Exposure-response relationships demonstrated near-maximal effect following the administration of >15 mg/kg. The mathematical model showed that exposures in the kidney were 5.77 times higher than those in plasma, and there was persistence of the drug at this site despite concentrations in plasma falling to undetectable levels. The in vitro and in vivo postantifungal effects were 2 to 5 and 8.41 h, respectively. The area under the concentration-time curve (AUC)/MIC ratio was the parameter that optimally linked drug exposure with the observed antifungal effect. The total drug AUC/MIC ratios associated with a 90% probability of survival in temporarily and persistently neutropenic mice were 270 and 670, respectively. Once corrected for protein binding, these values are similar to the magnitude of drug exposure associated with a high probability of a successful therapeutic outcome for other triazoles. This study provides the experimental foundation for the use of isavuconazole in patients with disseminated candidiasis.
Figures

References
-
- Cornely, O., A. Bohme, M. Heep, A. Schmidt-Hoffmann, and A. J. Ullmann. 2008. Pharmacokinetics, safety and tolerability results of a dose escalation study of isavuconazole in neutropenic patients, abstr. M-2137. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

