Phase II study of sorafenib in patients with metastatic or recurrent sarcomas
- PMID: 19451436
- PMCID: PMC2716936
- DOI: 10.1200/JCO.2008.20.4495
Phase II study of sorafenib in patients with metastatic or recurrent sarcomas
Abstract
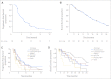
PURPOSE Since activity of sorafenib was observed in sarcoma patients in a phase I study, we performed a multicenter phase II study of daily oral sorafenib in patients with recurrent or metastatic sarcoma. PATIENTS AND METHODS We employed a multiarm study design, each representing a sarcoma subtype with its own Simon optimal two-stage design. In each arm, 12 patients who received 0 to 1 prior lines of therapy were treated (0 to 3 for angiosarcoma and malignant peripheral-nerve sheath tumor). If at least one Response Evaluation Criteria in Solid Tumors (RECIST) was observed, 25 further patients with that sarcoma subtype were accrued. Results Between October 2005 and November 2007, 145 patients were treated; 144 were eligible for toxicity and 122 for response. Median age was 55 years; female-male ratio was 1.8:1. The median number of cycles was 3. Five of 37 patients with angiosarcoma had a partial response (response rate, 14%). This was the only arm to meet the RECIST response rate primary end point. Median progression-free survival was 3.2 months; median overall survival was 14.3 months. Adverse events (typically dermatological) necessitated dose reduction for 61% of patients. Statistical modeling in this limited patient cohort indicated sorafenib toxicity was correlated inversely to patient height. There was no correlation between phosphorylated extracellular signal regulated kinase expression and response in six patients with angiosarcoma with paired pre- and post-therapy biopsies. CONCLUSION As a single agent, sorafenib has activity against angiosarcoma and minimal activity against other sarcomas. Further evaluation of sorafenib in these and possibly other sarcoma subtypes appears warranted, presumably in combination with cytotoxic or kinase-specific agents.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Brennan MF, Singer S, O'Sullivan B, et al. Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. ed 8. Philadelphia, PA: Wolters Kluwer; 2008. pp. 1741–1794.
-
- Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: A retrospective analysis. Int J Cancer. 2006;119:706–711. - PubMed
-
- Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol. 2002;20:2824–2831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

