Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience
- PMID: 19451444
- PMCID: PMC2717756
- DOI: 10.1200/JCO.2008.19.3789
Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience
Abstract
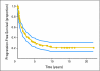
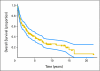
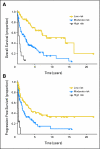
PURPOSE Primary CNS lymphoma (PCNSL) is confined to the CNS and/or the eyes at presentation and is usually initially treated with intravenous methotrexate-based chemotherapy and whole-brain radiotherapy (WBRT). However, the intact blood-brain barrier (BBB) can limit diffusion of methotrexate into brain and tumor. With BBB disruption (BBBD), enhanced drug delivery to the tumor can be achieved. PATIENTS AND METHODS This report summarizes the multi-institutional experience of 149 newly diagnosed (with no prior WBRT) patients with PCNSL treated with osmotic BBBD and intra-arterial (IA) methotrexate at four institutions from 1982 to 2005. In this series, 47.6% of patients were age > or = 60 years, and 42.3% had Karnofsky performance score (KPS) less than 70 at diagnosis. Results The overall response rate was 81.9% (57.8% complete; 24.2% partial). Median overall survival (OS) was 3.1 years (25% estimated survival at 8.5 years). Median progression-free survival (PFS) was 1.8 years, with 5-year PFS of 31% and 7-year PFS of 25%. In low-risk patients (age < 60 years and KPS > or = 70), median OS was approximately 14 years, with a plateau after approximately 8 years. Procedures were generally well tolerated; focal seizures (9.2%) were the most frequent side effect and lacked long-term sequelae. CONCLUSION This large series of patients treated over a 23-year period demonstrates that BBBD/IA methotrexate-based chemotherapy results in successful and durable tumor control and outcomes that are comparable or superior to other PCNSL treatment regimens.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Panageas KS, Elkin EB, DeAngelis LM, et al. Trends in survival from primary central nervous system lymphoma, 1975-1999: A population-based analysis. Cancer. 2005;104:2466–2472. - PubMed
-
- Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: A reality check. J Clin Oncol. 2007;25:2295–2305. - PubMed
-
- Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. - PubMed
-
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. - PubMed
-
- Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: The next step. J Clin Oncol. 2000;18:3144–3150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

