CCL21 mediates CD4+ T-cell costimulation via a DOCK2/Rac-dependent pathway
- PMID: 19451552
- PMCID: PMC2713469
- DOI: 10.1182/blood-2009-01-200923
CCL21 mediates CD4+ T-cell costimulation via a DOCK2/Rac-dependent pathway
Abstract
CD4(+) T cells use the chemokine receptor CCR7 to home to and migrate within lymphoid tissue, where T-cell activation takes place. Using primary T-cell receptor (TCR)-transgenic (tg) CD4(+) T cells, we explored the effect of CCR7 ligands, in particular CCL21, on T-cell activation. We found that the presence of CCL21 during early time points strongly increased in vitro T-cell proliferation after TCR stimulation, correlating with increased expression of early activation markers. CCL21 costimulation resulted in increased Ras- and Rac-GTP formation and enhanced phosphorylation of Akt, MEK, and ERK but not p38 or JNK. Kinase-dead PI3Kdelta(D910A/D910A) or PI3Kgamma-deficient TCR-tg CD4(+) T cells showed similar responsiveness to CCL21 costimulation as control CD4(+) T cells. Conversely, deficiency in the Rac guanine exchange factor DOCK2 significantly impaired CCL21-mediated costimulation in TCR-tg CD4(+) T cells, concomitant with impaired Rac- but not Ras-GTP formation. Using lymph node slices for live monitoring of T-cell behavior and activation, we found that G protein-coupled receptor signaling was required for early CD69 expression but not for Ca(2+) signaling. Our data suggest that the presence of CCL21 during early TCR signaling lowers the activation threshold through Ras- and Rac-dependent pathways leading to increased ERK phosphorylation.
Figures
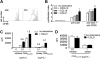


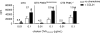


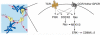
References
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. - PubMed
-
- Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. - PubMed
-
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. - PubMed
-
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. - PubMed
-
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

