Incorporating nucleosomes into thermodynamic models of transcription regulation
- PMID: 19451592
- PMCID: PMC2720181
- DOI: 10.1101/gr.088260.108
Incorporating nucleosomes into thermodynamic models of transcription regulation
Abstract
Transcriptional control is central to many cellular processes, and, consequently, much effort has been devoted to understanding its underlying mechanisms. The organization of nucleosomes along promoter regions is important for this process, since most transcription factors cannot bind nucleosomal sequences and thus compete with nucleosomes for DNA access. This competition is governed by the relative concentrations of nucleosomes and transcription factors and by their respective sequence binding preferences. However, despite its importance, a mechanistic understanding of the quantitative effects that the competition between nucleosomes and factors has on transcription is still missing. Here we use a thermodynamic framework based on fundamental principles of statistical mechanics to explore theoretically the effect that different nucleosome organizations along promoters have on the activation dynamics of promoters in response to varying concentrations of the regulating factors. We show that even simple landscapes of nucleosome organization reproduce experimental results regarding the effect of nucleosomes as general repressors and as generators of obligate binding cooperativity between factors. Our modeling framework also allows us to characterize the effects that various sequence elements of promoters have on the induction threshold and on the shape of the promoter activation curves. Finally, we show that using only sequence preferences for nucleosomes and transcription factors, our model can also predict expression behavior of real promoter sequences, thereby underscoring the importance of the interplay between nucleosomes and factors in determining expression kinetics.
Figures


 ) from transcription factor binding is set to 860 for a strong site, and 200 for a weak site, in accordance with the 4.3 ratio between strong and weak Pho4p sites reported in Lam et al. (2008).
) from transcription factor binding is set to 860 for a strong site, and 200 for a weak site, in accordance with the 4.3 ratio between strong and weak Pho4p sites reported in Lam et al. (2008).
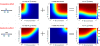

 ) from binding is set to 1200 for a high-affinity site, and 200 for a low-affinity site. (F) Shown is the ratio between the probability of transcription factor binding to the left site (marked as site 1) in a promoter with two transcription factor sites (Pbound,2sites) and in a promoter with one transcription factor site (Pbound,1site) at increasing transcription factor concentrations. Note that the cooperative effect between a low-affinity site and a high-affinity site is larger than the cooperative effect between two high-affinity sites.
) from binding is set to 1200 for a high-affinity site, and 200 for a low-affinity site. (F) Shown is the ratio between the probability of transcription factor binding to the left site (marked as site 1) in a promoter with two transcription factor sites (Pbound,2sites) and in a promoter with one transcription factor site (Pbound,1site) at increasing transcription factor concentrations. Note that the cooperative effect between a low-affinity site and a high-affinity site is larger than the cooperative effect between two high-affinity sites.
 ) from binding is set to 1200 for a high-affinity site, and 200 for a low-affinity site. (C) The ratio between Pbound,2sites to Pbound,1site from B at increasing transcription factor concentrations. This ratio represents the strength of the cooperative/destructive binding effect between the transcription factors. The ratio obtained is >1, indicating a positive cooperative effect. However, the strength of the effect depends on the locations of the sites relative to the boundary: the ratio is higher for sites that are relatively covered by nucleosomes at low transcription factor concentrations.
) from binding is set to 1200 for a high-affinity site, and 200 for a low-affinity site. (C) The ratio between Pbound,2sites to Pbound,1site from B at increasing transcription factor concentrations. This ratio represents the strength of the cooperative/destructive binding effect between the transcription factors. The ratio obtained is >1, indicating a positive cooperative effect. However, the strength of the effect depends on the locations of the sites relative to the boundary: the ratio is higher for sites that are relatively covered by nucleosomes at low transcription factor concentrations.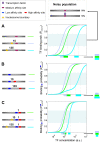

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
