Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer
- PMID: 19451693
- PMCID: PMC2689133
- DOI: 10.1172/JCI37480
Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer
Abstract
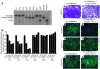
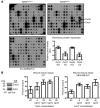
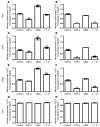
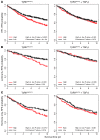
In human breast cancer, loss of carcinoma cell-specific response to TGF-beta signaling has been linked to poor patient prognosis. However, the mechanisms through which TGF-beta regulates these processes remain largely unknown. In an effort to address this issue, we have now identified gene expression signatures associated with the TGF-beta signaling pathway in human mammary carcinoma cells. The results strongly suggest that TGF-beta signaling mediates intrinsic, stromal-epithelial, and host-tumor interactions during breast cancer progression, at least in part, by regulating basal and oncostatin M-induced CXCL1, CXCL5, and CCL20 chemokine expression. To determine the clinical relevance of our results, we queried our TGF-beta-associated gene expression signatures in 4 human breast cancer data sets containing a total of 1,319 gene expression profiles and associated clinical outcome data. The signature representing complete abrogation of TGF-beta signaling correlated with reduced relapse-free survival in all patients; however, the strongest association was observed in patients with estrogen receptor-positive (ER-positive) tumors, specifically within the luminal A subtype. Together, the results suggest that assessment of TGF-beta signaling pathway status may further stratify the prognosis of ER-positive patients and provide novel therapeutic approaches in the management of breast cancer.
Figures

References
-
- Sun L., et al. Expression of transforming growth factor beta type II receptor leads to reduced malignancy in human breast cancer MCF-7 cells. J. Biol. Chem. 1994;269:26449–26455. - PubMed

