A phase II study of flavopiridol (Alvocidib) in combination with docetaxel in refractory, metastatic pancreatic cancer
- PMID: 19451750
- PMCID: PMC4053191
- DOI: 10.1159/000187135
A phase II study of flavopiridol (Alvocidib) in combination with docetaxel in refractory, metastatic pancreatic cancer
Abstract
Background/aims: Pancreatic adenocarcinoma (PC) harbors frequent alterations in p16, resulting in cell cycle dysregulation. A phase I study of docetaxel and flavopiridol, a pan-cyclin-dependent kinase inhibitor, demonstrated encouraging clinical activity in PC. This phase II study was designed to further define the efficacy and toxicity of this regimen in patients with previously treated PC.
Methods: Patients with gemcitabine-refractory, metastatic PC were treated with docetaxel 35 mg/m(2) followed by flavopiridol 80 mg/m(2) on days 1, 8, and 15 of a 28-day cycle. Tumor measurements were performed every two cycles. A Simon two-stage design was used to evaluate the primary endpoint of response.
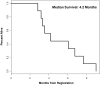
Results: Ten patients were enrolled, and 9 were evaluable for response. No objective responses were observed; however, 3 patients (33%) achieved transient stable disease, with one of these patients achieving a 20% reduction in tumor size. Median survival was 4.2 months, with no patients alive at the time of analysis. Adverse events were significant, with 7 patients (78%) requiring >or=1 dose reduction for transaminitis (11%), grade 4 neutropenia (33%), grade 3 fatigue (44%), and grade 3 diarrhea (22%).
Conclusions: The combination of flavopiridol and docetaxel has minimal activity and significant toxicity in this patient population. These results reflect the challenges of treating patients with PC in a second-line setting where the risk/benefit equation is tightly balanced.
Copyright 2009 S. Karger AG, Basel.
Similar articles
-
Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer.Clin Cancer Res. 2004 Aug 1;10(15):5038-47. doi: 10.1158/1078-0432.CCR-04-0025. Clin Cancer Res. 2004. PMID: 15297405 Clinical Trial.
-
Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors.Clin Cancer Res. 2007 Oct 1;13(19):5841-6. doi: 10.1158/1078-0432.CCR-07-1218. Clin Cancer Res. 2007. PMID: 17908977 Clinical Trial.
-
Salvage chemotherapy with mitomycin, docetaxel, and irinotecan (MDI regimen) in metastatic pancreatic adenocarcinoma: a phase I and II trial.Cancer Invest. 2004;22(5):688-96. doi: 10.1081/cnv-200032929. Cancer Invest. 2004. PMID: 15581049 Clinical Trial.
-
A phase I study of flavopiridol and docetaxel.Invest New Drugs. 2006 Jul;24(4):305-10. doi: 10.1007/s10637-005-4343-5. Invest New Drugs. 2006. PMID: 16683073 Clinical Trial.
-
Second-line chemotherapy with capecitabine (Xeloda) and docetaxel (Taxotere) in previously treated, unresectable adenocarcinoma of pancreas: the final results of a phase II trial.Cancer Chemother Pharmacol. 2011 Feb;67(2):361-8. doi: 10.1007/s00280-010-1329-6. Epub 2010 Apr 29. Cancer Chemother Pharmacol. 2011. PMID: 20428874 Clinical Trial.
Cited by
-
A dose-finding, pharmacokinetic and pharmacodynamic study of a novel schedule of flavopiridol in patients with advanced solid tumors.Invest New Drugs. 2012 Apr;30(2):629-38. doi: 10.1007/s10637-010-9563-7. Epub 2010 Oct 12. Invest New Drugs. 2012. PMID: 20938713 Free PMC article. Clinical Trial.
-
Fascaplysin as a specific inhibitor for CDK4: insights from molecular modelling.PLoS One. 2012;7(8):e42612. doi: 10.1371/journal.pone.0042612. Epub 2012 Aug 14. PLoS One. 2012. PMID: 22905154 Free PMC article.
-
Cyclin-dependent protein kinases and cell cycle regulation in biology and disease.Signal Transduct Target Ther. 2025 Jan 13;10(1):11. doi: 10.1038/s41392-024-02080-z. Signal Transduct Target Ther. 2025. PMID: 39800748 Free PMC article. Review.
-
The CDK inhibitors in cancer research and therapy.J Cancer Res Clin Oncol. 2011 Oct;137(10):1409-18. doi: 10.1007/s00432-011-1039-4. Epub 2011 Aug 30. J Cancer Res Clin Oncol. 2011. PMID: 21877198 Free PMC article. Review.
-
Phase II clinical trials on investigational drugs for the treatment of pancreatic cancers.Expert Opin Investig Drugs. 2015 Jun;24(6):781-94. doi: 10.1517/13543784.2015.1026963. Epub 2015 Mar 25. Expert Opin Investig Drugs. 2015. PMID: 25809274 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Singh M, Maitra A. Precursor lesions of pancreatic cancer: Molecular pathology and clinical implications. Pancreatology. 2007;7:9–19. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase iii trial of the national cancer institute of canada clinical trials group. J Clin Oncol. 2007;25:1960–1966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical