A physiologically realistic in vitro model of microvascular networks
- PMID: 19452279
- PMCID: PMC2888702
- DOI: 10.1007/s10544-009-9322-8
A physiologically realistic in vitro model of microvascular networks
Abstract
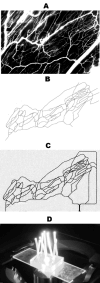
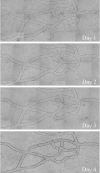
Existing microfluidic devices, e.g. parallel plate flow chambers, do not accurately depict the geometry of microvascular networks in vivo. We have developed a synthetic microvascular network (SMN) on a polydimethalsiloxane (PDMS) chip that can serve as an in vitro model of the bifurcations, tortuosities, and cross-sectional changes found in microvascular networks in vivo. Microvascular networks from a cremaster muscle were mapped using a modified Geographical Information System, and then used to manufacture the SMNs on a PDMS chip. The networks were cultured with bovine aortic endothelial cells (BAEC), which reached confluency 3-4 days after seeding. Propidium iodide staining indicated viable and healthy cells showing normal behavior in these networks. Anti-ICAM-1 conjugated 2-mum microspheres adhered to BAEC cells activated with TNF-alpha in significantly larger numbers compared to control IgG conjugated microspheres. This preferential adhesion suggests that cultured cells retain an intact cytokine response in the SMN. This microfluidic system can provide novel insight into characterization of drug delivery particles and dynamic flow conditions in microvascular networks.
Figures



References
-
- Camp JP, Stokol T, Shuler ML. Biomed Microdevices. 2007;10(2) PMCID: 17891456. - PubMed
-
- Cokelet GR, Soave R, Pugh G, Rathbun L. Microvasc Res. 1993;46(3) PMCID: 8121322. - PubMed
-
- Decuzzi P, Ferrari M. Biomaterials. 2008;29(3) PMCID: 17936897. - PubMed
-
- El Ali J, Sorger PK, Jensen KF. Nature. 2006;442(7101) PMCID: 16871208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

