Handling data below the limit of quantification in mixed effect models
- PMID: 19452283
- PMCID: PMC2691472
- DOI: 10.1208/s12248-009-9112-5
Handling data below the limit of quantification in mixed effect models
Abstract
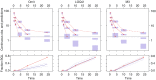
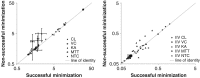
The purpose of this study is to investigate the impact of observations below the limit of quantification (BQL) occurring in three distinctly different ways and assess the best method for prevention of bias in parameter estimates and for illustrating model fit using visual predictive checks (VPCs). Three typical ways in which BQL can occur in a model was investigated with simulations from three different models and different levels of the limit of quantification (LOQ). Model A was used to represent a case with BQL observations in an absorption phase of a PK model whereas model B represented a case with BQL observations in the elimination phase. The third model, C, an indirect response model illustrated a case where the variable of interest in some cases decreases below the LOQ before returning towards baseline. Different approaches for handling of BQL data were compared with estimation of the full dataset for 100 simulated datasets following models A, B, and C. An improved standard for VPCs was suggested to better evaluate simulation properties both for data above and below LOQ. Omission of BQL data was associated with substantial bias in parameter estimates for all tested models even for seemingly small amounts of censored data. Best performance was seen when the likelihood of being below LOQ was incorporated into the model. In the tested examples this method generated overall unbiased parameter estimates. Results following substitution of BQL observations with LOQ/2 were in some cases shown to introduce bias and were always suboptimal to the best method. The new standard VPCs was found to identify model misfit more clearly than VPCs of data above LOQ only.
Figures




References
-
- FDA guidance for industry bioanalytical method validation. http://www.fdagov/cder/guidance/4252fnl.pdf (accessed 05/05/2007).
-
- Bergstrand M, Plan E, Kjellsson M, Karlsson MO. A comparison of methods for handling of data below the limit of quantification in NONMEM VI. PAGE 16 (2007) Abstr 1201 [www.page-meeting.org/?abstract=1201].
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

