Oligonucleotide microchip for subtyping of influenza A virus
- PMID: 19453417
- PMCID: PMC4941880
- DOI: 10.1111/j.1750-2659.2007.00018.x
Oligonucleotide microchip for subtyping of influenza A virus
Abstract
Background: Influenza A viruses are classified into subtypes depending on the antigenic properties of their two outer glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Sixteen subtypes of HA and nine of NA are known. Lately, the circulation of some subtypes (H7N7, H5N1) has been closely watched because of the epidemiological threat they present.
Objectives: This study assesses the potential of using gel-based microchip technology for fast and sensitive molecular subtyping of the influenza A virus.
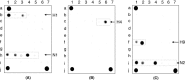
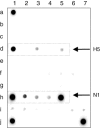
Methods: The method employs a microchip of 3D gel-based elements containing immobilized probes. Segments of the HA and NA genes are amplified using multiplex RT-PCR and then hybridized with the microchip.
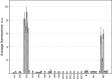
Results: The developed microchip was validated using a panel of 21 known reference strains of influenza virus. Selected strains represented different HA and NA subtypes derived from avian, swine and human hosts. The whole procedure takes 10 hours and enables one to identify 15 subtypes of HA and two subtypes of NA. Forty-one clinical samples isolated during the poultry fall in Novosibirsk (Russia, 2005) were successfully identified using the proposed technique. The sensitivity and specificity of the method were 76% and 100%, respectively, compared with the 'gold standard' techniques (virus isolation with following characterization by immunoassay).
Conclusions: We conclude that the method of subtyping using gel-based microchips is a promising approach for fast detection and identification of influenza A, which may greatly improve its monitoring.
Figures


Similar articles
-
SYBR green-based real-time reverse transcription-PCR for typing and subtyping of all hemagglutinin and neuraminidase genes of avian influenza viruses and comparison to standard serological subtyping tests.J Clin Microbiol. 2012 Jan;50(1):37-45. doi: 10.1128/JCM.01195-11. Epub 2011 Oct 26. J Clin Microbiol. 2012. PMID: 22031706 Free PMC article.
-
[Oligonucleotide microarray for subtyping avian influenza virus].Wei Sheng Wu Xue Bao. 2008 Sep;48(9):1241-9. Wei Sheng Wu Xue Bao. 2008. PMID: 19062651 Chinese.
-
Rapid and highly sensitive neuraminidase subtyping of avian influenza viruses by use of a diagnostic DNA microarray.J Clin Microbiol. 2009 Sep;47(9):2985-8. doi: 10.1128/JCM.00850-09. Epub 2009 Jul 8. J Clin Microbiol. 2009. PMID: 19587298 Free PMC article.
-
Recent zoonoses caused by influenza A viruses.Rev Sci Tech. 2000 Apr;19(1):197-225. doi: 10.20506/rst.19.1.1220. Rev Sci Tech. 2000. PMID: 11189716 Review.
-
Molecular evolution of hemagglutinin gene of Influenza A virus.Front Biosci (Schol Ed). 2018 Jan 1;10(1):101-118. doi: 10.2741/s502. Front Biosci (Schol Ed). 2018. PMID: 28930520 Review.
Cited by
-
Development of DNA Microarray for Parallel Detection of Community-Acquired Pneumonia Bacterial Pathogens.Sovrem Tekhnologii Med. 2024;16(2):16-26. doi: 10.17691/stm2024.16.2.02. Epub 2024 Apr 27. Sovrem Tekhnologii Med. 2024. PMID: 39539749 Free PMC article.
-
Identification of genetic determinants of influenza A virus resistance to adamantanes and neuraminidase inhibitors using biological microarray.Dokl Biochem Biophys. 2015;460:4-8. doi: 10.1134/S1607672915010032. Epub 2015 Mar 13. Dokl Biochem Biophys. 2015. PMID: 25772979 No abstract available.
-
Universal oligonucleotide microarray for sub-typing of Influenza A virus.PLoS One. 2011 Apr 29;6(4):e17529. doi: 10.1371/journal.pone.0017529. PLoS One. 2011. PMID: 21559081 Free PMC article.
-
Comparative evaluation of effectiveness of IAVchip DNA microarray in influenza A diagnosis.ScientificWorldJournal. 2014;2014:620580. doi: 10.1155/2014/620580. Epub 2014 Nov 23. ScientificWorldJournal. 2014. PMID: 25548788 Free PMC article.
-
Design of a set of probes with high potential for influenza virus epidemiological surveillance.Bioinformation. 2013 Apr 30;9(8):414-20. doi: 10.6026/97320630009414. Print 2013. Bioinformation. 2013. PMID: 23750091 Free PMC article.
References
-
- Paget WJ, Meerhoff TJ, Meijer A. Epidemiological and virological assessment of influenza activity in Europe during the 2003–2004 season. Euro Surveill 2005;11:107–111. - PubMed
-
- World Health Organization . H5N1 avian influenza: timeline of major events [WWW document]. URL http://www.who.int/csr/disease/avian_influenza/timeline_2007_04_20.pdf [accessed 20 April 2007].
-
- World Health Organization . Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO [WWW document]. URL http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_... [accessed 11 April 2007].
-
- World Organization for Animal Health . Update on avian influenza in animals [WWW document]. URL http://www.oie.int/downld/AVIAN%20INFLUENZA/A_AI‐Asia.htm [accessed 20 April 2007].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

