Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs
- PMID: 19453471
- PMCID: PMC4941893
- DOI: 10.1111/j.1750-2659.2008.00038.x
Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs
Abstract
Background: For protection against (re-)infection by influenza virus not only the magnitude of the immune response but also its quality in terms of antibody subclass and T helper profile is important. Information about the type of immune response elicited by vaccination is therefore urgently needed.
Objectives: The aim of the study was to evaluate in detail the immune response elicited by three current influenza vaccine formulations and to shed light on vaccine characteristics which determine this response.
Methods: Mice were immunized with whole inactivated virus (WIV), virosomes (VS) or subunit vaccine (SU). Following subsequent infection with live virus, serum antibody titers and Th cell responses were measured. The effects of the vaccines on cytokine production by conventional and plasmacytoid dendritic cells were investigated in vitro.
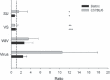
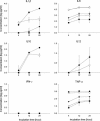
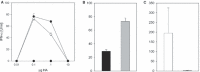
Results and conclusions: In Balb/c mice (Th2 prone) as well as in C57Bl/6 mice (Th1 prone), WIV induced consistently higher hemagglutination-inhibition titers and virus-neutralizing antibody titers than VS or SU. In contrast to VS and SU, WIV stimulated the production of the antibody subclasses IgG2a (Balb/c) and IgG2c (C57BL/6), considered to be particularly important for viral clearance, and activation of IFN-gamma-producing T cells. Similar to live virus, WIV stimulated the production of proinflammatory cytokines by conventional dendritic cells and IFN-alpha by plasmacytoid cells, while VS and SU had little effect on cytokine synthesis by either cell type. We conclude that vaccination with WIV in contrast to VS or SU results in the desired Th1 response presumably by induction of type I interferon and other proinflammatory cytokines.
Figures



References
-
- Stöhr K, Kieny M, Wood D. Influenza pandemic vaccines: how to ensure a low‐cost, low‐dose option. Nat Rev Micro 2006; 4:565–566. - PubMed
-
- World Health Organization . Avian influenza assessing the pandemic threat, 2005. Available at: http://www.who.int/crs/desease/infuenza/WHO_CDS_2005_29/en/.
-
- Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet 2006; 368:2211–2218. - PubMed
-
- Wilschut JC, McElhaney JE, Palache AM. Vaccination: cornerstone of influenza control In: Wilschut JC, McElhaney JE, Palache AM, eds. Influenza. Amsterdam: Elsevier, 2006; 150–183.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

