Modulation of the keratinocyte-fibroblast paracrine relationship with gelatin-based semi-interpenetrating networks containing bioactive factors for wound repair
- PMID: 19454166
- PMCID: PMC3757500
- DOI: 10.1163/156856209X444402
Modulation of the keratinocyte-fibroblast paracrine relationship with gelatin-based semi-interpenetrating networks containing bioactive factors for wound repair
Abstract
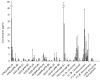
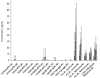
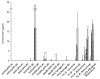
Gelatin-based semi-interpenetrating networks (sIPNs) containing soluble and covalently-linked bioactive factors have been shown to aid in wound healing; however, the biological responses elicited by the introduction of sIPN biomaterials remain unclear. In the current study, modulation of the re-epithelialization phase of wound healing by sIPNs grafted with PEGylated fibronectin-derived peptides and utilized as platforms for the delivery of exogenous keratinocyte growth factor (KGF) was evaluated. Following wounding, keratinocyte migration, proliferation and protein secretion is largely controlled by diffusible factors, such as KGF, released by the underlying fibroblasts. The impact of sIPNs and exogenous KGF upon the latter keratinocyte-fibroblast paracrine relationship and keratinocyte behavior was explored by monitoring keratinocyte adhesion and cytokine (IL-1alpha, IL-1beta, IL-6, KGF, GM-CSF and TGF-alpha) release. Results were generally similar for keratinocyte monoculture and keratinocyte-fibroblast co-culture systems. Although keratinocyte adhesion increased over time for positive control surfaces, adhesion to the sIPNs remained low throughout the course of the study. Release of IL-1alpha and GM-CSF was increased by exogenous KGF. The effects were more noticeable on the positive control surfaces relative to the sIPN surfaces. Regulation of the release of TGF-alpha was surface dependent, while IL-6 release was dependent upon surface type, the inclusion of exogenous KGF and the presence of fibroblasts. The findings indicate that during re-epithelialization, sIPNs containing soluble bioactive factors aid in wound healing primarily by serving as conduits for KGF, which induces the release of other key cytokines involved in tissue repair.
Figures



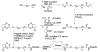

Similar articles
-
Keratinocyte-fibroblast paracrine interaction: the effects of substrate and culture condition.Biomaterials. 2005 Jun;26(17):3673-82. doi: 10.1016/j.biomaterials.2004.09.054. Biomaterials. 2005. PMID: 15621258
-
The effects of TGF-alpha, IL-1beta and PDGF on fibroblast adhesion to ECM-derived matrix and KGF gene expression.Biomaterials. 2010 Mar;31(9):2542-8. doi: 10.1016/j.biomaterials.2009.12.018. Epub 2009 Dec 29. Biomaterials. 2010. PMID: 20036421 Free PMC article.
-
Fibroblasts regulate monocyte response to ECM-derived matrix: the effects on monocyte adhesion and the production of inflammatory, matrix remodeling, and growth factor proteins.J Biomed Mater Res A. 2009 Jun 15;89(4):841-53. doi: 10.1002/jbm.a.32431. J Biomed Mater Res A. 2009. PMID: 19437738 Free PMC article.
-
Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization.Tissue Eng Part B Rev. 2022 Jun;28(3):665-676. doi: 10.1089/ten.TEB.2021.0030. Epub 2021 Oct 18. Tissue Eng Part B Rev. 2022. PMID: 34238035 Review.
-
An overview on keratinocyte growth factor: from the molecular properties to clinical applications.Protein Pept Lett. 2014 Mar;21(3):306-17. doi: 10.2174/09298665113206660115. Protein Pept Lett. 2014. PMID: 24188496 Review.
Cited by
-
[New developments in skin reconstruction - cell cultures and skin substitutes plus review of the literature].Ann Burns Fire Disasters. 2010 Sep 30;23(3):131-6. Ann Burns Fire Disasters. 2010. PMID: 21991212 Free PMC article. French.
-
Identification of regulatory Hck and PAI-2 proteins in the monocyte response to PEG-containing matrices.Biomaterials. 2009 Aug;30(23-24):3825-33. doi: 10.1016/j.biomaterials.2009.04.007. Epub 2009 May 14. Biomaterials. 2009. PMID: 19443025 Free PMC article.
-
A two-layer skin construct consisting of a collagen hydrogel reinforced by a fibrin-coated polylactide nanofibrous membrane.Int J Nanomedicine. 2019 Jul 8;14:5033-5050. doi: 10.2147/IJN.S200782. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31371945 Free PMC article.
-
Ex vivo culture of keratinocytes on papillary and reticular dermal layers remodels skin explants differently: towards improved wound care.Arch Dermatol Res. 2019 Oct;311(8):647-652. doi: 10.1007/s00403-019-01941-w. Epub 2019 Jun 5. Arch Dermatol Res. 2019. PMID: 31168656 Free PMC article.
-
Effect of Hypertrophic Scar Fibroblast-Derived Exosomes on Keratinocytes of Normal Human Skin.Int J Mol Sci. 2023 Mar 24;24(7):6132. doi: 10.3390/ijms24076132. Int J Mol Sci. 2023. PMID: 37047109 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
