Transgenic mice expressing a cameleon fluorescent Ca2+ indicator in astrocytes and Schwann cells allow study of glial cell Ca2+ signals in situ and in vivo
- PMID: 19454294
- PMCID: PMC3142666
- DOI: 10.1016/j.jneumeth.2009.05.006
Transgenic mice expressing a cameleon fluorescent Ca2+ indicator in astrocytes and Schwann cells allow study of glial cell Ca2+ signals in situ and in vivo
Abstract
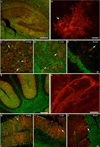
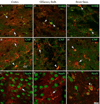
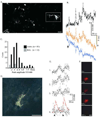
Glial cell Ca2+ signals play a key role in glial-neuronal and glial-glial network communication. Numerous studies have thus far utilized cell-permeant and injected Ca2+ indicator dyes to investigate glial Ca2+ signals in vitro and in situ. Genetically encoded fluorescent Ca2+ indicators have emerged as novel probes for investigating cellular Ca2+ signals. We have expressed one such indicator protein, the YC 3.60 cameleon, under the control of the S100beta promoter and directed its expression predominantly in astrocytes and Schwann cells. Expression of YC 3.60 extended into the entire cellular cytoplasmic compartment and the fine terminal processes of protoplasmic astrocytes and Schwann cell Cajal bands. In the brain, all the cells known to express S100beta in the adult or during development, expressed YC 3.60. While expression was most extensive in astrocytes, other glial cell types that express S100beta, such as NG2 and CNP-positive oligodendrocyte progenitor cells (OP cells), microglia, and some of the large motor neurons in the brain stem, also contained YC 3.60 fluorescence. Using a variety of known in situ and in vivo assays, we found that stimuli known to elicit Ca2+ signals in astrocytes caused substantial and rapid Ca2+ signals in the YC 3.60-expressing astrocytes. In addition, forepaw stimulation while imaging astrocytes through a cranial window in the somatosensory cortex in live mice, revealed robust evoked and spontaneous Ca2+ signals. These results, for the first time, show that genetically encoded reporter is capable of recording activity-dependent Ca2+ signals in the astrocyte processes, and networks.
Figures



Similar articles
-
In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator.Cell Rep. 2014 Jul 10;8(1):311-8. doi: 10.1016/j.celrep.2014.05.056. Epub 2014 Jun 26. Cell Rep. 2014. PMID: 24981861
-
Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons.J Neurosci. 2004 Jan 21;24(3):722-32. doi: 10.1523/JNEUROSCI.2859-03.2004. J Neurosci. 2004. PMID: 14736858 Free PMC article.
-
Stimulation-evoked Ca2+ signals in astrocytic processes at hippocampal CA3-CA1 synapses of adult mice are modulated by glutamate and ATP.J Neurosci. 2015 Feb 18;35(7):3016-21. doi: 10.1523/JNEUROSCI.3319-14.2015. J Neurosci. 2015. PMID: 25698739 Free PMC article.
-
Recent developments in the understanding of astrocyte function in the cerebellum in vivo.Cerebellum. 2010 Sep;9(3):264-71. doi: 10.1007/s12311-009-0139-z. Cerebellum. 2010. PMID: 19904577 Review.
-
Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells.J Mol Med (Berl). 1997 Sep;75(9):653-63. doi: 10.1007/s001090050149. J Mol Med (Berl). 1997. PMID: 9351704 Review.
Cited by
-
Two-photon microscopy for chemical neuroscience.ACS Chem Neurosci. 2011 Apr 20;2(4):185-197. doi: 10.1021/cn100111a. ACS Chem Neurosci. 2011. PMID: 21731799 Free PMC article.
-
Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex.Nat Methods. 2012 Jun;9(6):597-602. doi: 10.1038/nmeth.2013. Epub 2012 May 6. Nat Methods. 2012. PMID: 22561989
-
Imaging calcium signals in vivo: a powerful tool in physiology and pharmacology.Br J Pharmacol. 2011 Aug;163(8):1605-25. doi: 10.1111/j.1476-5381.2010.00988.x. Br J Pharmacol. 2011. PMID: 20718728 Free PMC article. Review.
-
Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses.J Gen Physiol. 2013 May;141(5):633-47. doi: 10.1085/jgp.201210949. Epub 2013 Apr 15. J Gen Physiol. 2013. PMID: 23589582 Free PMC article.
-
Application of FRET Biosensors in Mechanobiology and Mechanopharmacological Screening.Front Bioeng Biotechnol. 2020 Nov 9;8:595497. doi: 10.3389/fbioe.2020.595497. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33240867 Free PMC article. Review.
References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. - PubMed
-
- Bushong EA, Martone ME, Ellisman MH. Examination of the relationship between astrocyte morphology and laminar boundaries in the molecular layer of adult dentate gyrus. J Comp Neurol. 2003;462:241–251. - PubMed
-
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. - PubMed
-
- Diez-Garcia J, Matsushita S, Mutoh H, Nakai J, Ohkura M, Yokoyama J, Dimitrov D, Knopfel T. Activation of cerebellar parallel fibers monitored in transgenic mice expressing a fluorescent Ca2+ indicator protein. Eur J Neurosci. 2005;22:627–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

