Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I
- PMID: 19454348
- PMCID: PMC2737813
- DOI: 10.1016/j.chom.2009.04.006
Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I
Abstract
The ubiquitin ligase TRIM25 mediates Lysine 63-linked ubiquitination of the N-terminal CARD domains of the viral RNA sensor RIG-I to facilitate type I interferon (IFN) production and antiviral immunity. Here, we report that the influenza A virus nonstructural protein 1 (NS1) specifically inhibits TRIM25-mediated RIG-I CARD ubiquitination, thereby suppressing RIG-I signal transduction. A novel domain in NS1 comprising E96/E97 residues mediates its interaction with the coiled-coil domain of TRIM25, thus blocking TRIM25 multimerization and RIG-I CARD domain ubiquitination. Furthermore, a recombinant influenza A virus expressing an E96A/E97A NS1 mutant is defective in blocking TRIM25-mediated antiviral IFN response and loses virulence in mice. Our findings reveal a mechanism by which influenza virus inhibits host IFN response and also emphasize the vital role of TRIM25 in modulating antiviral defenses.
Figures

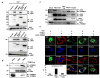

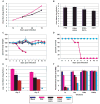
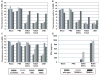
Comment in
-
Influenza A virus TRIMs the type I interferon response.Cell Host Microbe. 2009 May 8;5(5):420-1. doi: 10.1016/j.chom.2009.05.004. Cell Host Microbe. 2009. PMID: 19454344
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Albrecht RA, Garcia-Sastre A. Suppression of innate immunity by orthomyxoviruses. In: Brasier AR, Garcia-Sastre A, Lemon SM, editors. Cellular signalling and innate immune responses to RNA virus infections. Washington: ASM Press; 2009. pp. 267–315.
-
- Bornholdt ZA, Prasad BV. X-ray structure of influenza virus NS1 effector domain. Nat Struct Mol Biol. 2006;13:559–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DE019085/DE/NIDCR NIH HHS/United States
- R01 AI46954/AI/NIAID NIH HHS/United States
- R01 AI046954/AI/NIAID NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- R01 CA115284/CA/NCI NIH HHS/United States
- HHSN266200700010C/AI/NIAID NIH HHS/United States
- U54 AI57158/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- DE019085/DE/NIDCR NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- U19 AI62623/AI/NIAID NIH HHS/United States
- U19 AI083025/AI/NIAID NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- CA115284/CA/NCI NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
- U19 AI062623/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

