Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine
- PMID: 19454351
- PMCID: PMC2768556
- DOI: 10.1016/j.chom.2009.03.011
Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine
Abstract
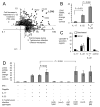
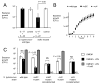
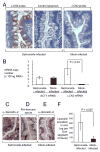
In response to enteric pathogens, the inflamed intestine produces antimicrobial proteins, a process mediated by the cytokines IL-17 and IL-22. Salmonella enterica serotype Typhimurium thrives in the inflamed intestinal environment, suggesting that the pathogen is resistant to antimicrobials it encounters in the intestinal lumen. However, the identity of these antimicrobials and corresponding bacterial resistance mechanisms remain unknown. Here, we report that enteric infection of rhesus macaques and mice with S. Typhimurium resulted in marked Il-17- and IL-22-dependent intestinal epithelial induction and luminal accumulation of lipocalin-2, an antimicrobial protein that prevents bacterial iron acquisition. Resistance to lipocalin-2, mediated by the iroBCDE iroN locus, conferred a competitive advantage to the bacterium in colonizing the inflamed intestine of wild-type but not of lipocalin-2-deficient mice. Thus, resistance to lipocalin-2 defines a specific adaptation of S. Typhimurium for growth in the inflamed intestine.
Figures




Comment in
-
A precious metal heist.Cell Host Microbe. 2009 May 8;5(5):422-4. doi: 10.1016/j.chom.2009.05.005. Cell Host Microbe. 2009. PMID: 19454345
References
-
- Ackermann M, Stecher B, Freed NE, Songhet P, Hardt WD, Doebeli M. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. J. Wiley & Sons; 1994.
-
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI040124/AI/NIAID NIH HHS/United States
- R01 AI050553/AI/NIAID NIH HHS/United States
- T32 AI60555/AI/NIAID NIH HHS/United States
- T32 AI060555/AI/NIAID NIH HHS/United States
- R01 AI076246/AI/NIAID NIH HHS/United States
- R01 AI044170/AI/NIAID NIH HHS/United States
- R29 AI040124/AI/NIAID NIH HHS/United States
- R01 DK043183/DK/NIDDK NIH HHS/United States
- R01 AI040124/AI/NIAID NIH HHS/United States
- R37 AI032738/AI/NIAID NIH HHS/United States
- AI42081/AI/NIAID NIH HHS/United States
- AI079173/AI/NIAID NIH HHS/United States
- DK43183/DK/NIDDK NIH HHS/United States
- DE018097/DE/NIDCR NIH HHS/United States
- R01 AI043274/AI/NIAID NIH HHS/United States
- R21 DE018097/DE/NIDCR NIH HHS/United States
- R01 AI050843/AI/NIAID NIH HHS/United States
- AI044170/AI/NIAID NIH HHS/United States
- AI50843/AI/NIAID NIH HHS/United States
- DK61297/DK/NIDDK NIH HHS/United States
- R01 DK061297/DK/NIDDK NIH HHS/United States
- AI050553/AI/NIAID NIH HHS/United States
- R21 AI079173/AI/NIAID NIH HHS/United States
- R01 AI042081/AI/NIAID NIH HHS/United States
- AI43274/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

