Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway
- PMID: 19454490
- PMCID: PMC2709465
- DOI: 10.1093/cvr/cvp153
Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway
Abstract
Aims: The presence and potential function of transient receptor potential melastatin 7 (TRPM7), a Ca2+-permeable non-selective cation channel of the TRP channel superfamily in human vascular endothelial cells, were examined.
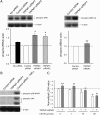
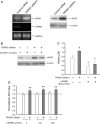
Methods and results: Whole-cell patch-clamp recordings showed outward-rectifying currents in human umbilical vein endothelial cells (HUVECs), which was potentiated by removing the extracellular Ca2+ and Mg2+, but inhibited by non-specific TRPM7 blocker Gd3+ or 2-aminoethoxydiphenyl borate (2-APB). TRPM7 mRNA was detected in HUVECs by RT-PCR, but TRPM6, its closest homologue, was not. Silencing TRPM7 by small interfering RNA (siRNA) decreased the level of TRPM7 mRNA and the TRPM7-like current. Interestingly, knockdown of TRPM7 with siRNA or inhibition of TRPM7 function with 2-APB increased the phosphorylation of extracellular signal-regulated kinase (ERK) and enhanced growth/proliferation of HUVECs. This enhanced cell growth/proliferation was abolished by an inhibitor of the ERK signalling pathway. In addition to cell growth/proliferation, silencing TRPM7 also increased expression of nitric oxide synthase and nitric oxide production in an ERK pathway-dependent manner.
Conclusion: These observations suggest that TRPM7 channels may play an important role in the function of vascular endothelial cells.
Figures




Similar articles
-
Role of TRPM7 channels in hyperglycemia-mediated injury of vascular endothelial cells.PLoS One. 2013 Nov 1;8(11):e79540. doi: 10.1371/journal.pone.0079540. eCollection 2013. PLoS One. 2013. PMID: 24223965 Free PMC article.
-
Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways.PLoS One. 2015 Mar 23;10(3):e0119912. doi: 10.1371/journal.pone.0119912. eCollection 2015. PLoS One. 2015. PMID: 25799367 Free PMC article.
-
TRPM7 regulates vascular endothelial cell adhesion and tube formation.Am J Physiol Cell Physiol. 2015 Feb 15;308(4):C308-18. doi: 10.1152/ajpcell.00275.2013. Epub 2014 Dec 3. Am J Physiol Cell Physiol. 2015. PMID: 25472964 Free PMC article.
-
Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension.Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1103-18. doi: 10.1152/ajpheart.00903.2007. Epub 2008 Jan 11. Am J Physiol Heart Circ Physiol. 2008. PMID: 18192217 Review.
-
TRPM7 and its role in neurodegenerative diseases.Channels (Austin). 2015;9(5):253-61. doi: 10.1080/19336950.2015.1075675. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218331 Free PMC article. Review.
Cited by
-
A bibliometric analysis and review of recent researches on TRPM7.Channels (Austin). 2020 Dec;14(1):203-215. doi: 10.1080/19336950.2020.1788355. Channels (Austin). 2020. PMID: 32643506 Free PMC article. Review.
-
Development and optimization of a high-throughput bioassay for TRPM7 ion channel inhibitors.J Biomol Screen. 2010 Jun;15(5):498-507. doi: 10.1177/1087057110368294. Epub 2010 Apr 22. J Biomol Screen. 2010. PMID: 20413646 Free PMC article.
-
Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels.J Biol Chem. 2010 Mar 5;285(10):7430-9. doi: 10.1074/jbc.M109.040485. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048154 Free PMC article.
-
Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways.Mar Drugs. 2015 Apr 22;13(4):2505-25. doi: 10.3390/md13042505. Mar Drugs. 2015. PMID: 25913706 Free PMC article.
-
MiR-9 promotes angiogenesis of endothelial progenitor cell to facilitate thrombi recanalization via targeting TRPM7 through PI3K/Akt/autophagy pathway.J Cell Mol Med. 2020 Apr;24(8):4624-4632. doi: 10.1111/jcmm.15124. Epub 2020 Mar 9. J Cell Mol Med. 2020. PMID: 32147957 Free PMC article.
References
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, et al. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. - PubMed
-
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

