Overexpression of PwTUA1, a pollen-specific tubulin gene, increases pollen tube elongation by altering the distribution of alpha-tubulin and promoting vesicle transport
- PMID: 19454597
- PMCID: PMC2692020
- DOI: 10.1093/jxb/erp143
Overexpression of PwTUA1, a pollen-specific tubulin gene, increases pollen tube elongation by altering the distribution of alpha-tubulin and promoting vesicle transport
Abstract
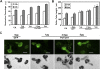
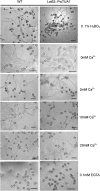
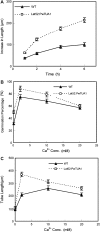
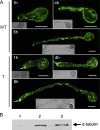
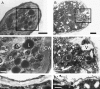
Tubulin genes are intimately associated with cell division and cell elongation, which are central to plant secondary cell wall development. However, their roles in pollen tube polar growth remain elusive. Here, a TUA1 gene from Picea wilsonii, which is specifically expressed in pollen, was isolated. Semi-quantitative RT-PCR analysis showed that the amount of PwTUA1 transcript varied at each stage of growth of the pollen tube and was induced by calcium ions and boron. Transient expression analysis in P. wilsonii pollen indicated that PwTUA1 improved pollen germination and pollen tube growth. The pollen of transgenic Arabidopsis overexpressing PwTUA1 also showed a higher percentage of germination and faster growth than wild-type plants not only in optimal germination medium, but also in medium supplemented with elevated levels of exogenous calcium ions or boron. Immunofluorescence and electron microscopy showed alpha-tubulin to be enriched and more vesicles accumulated in the apex region in germinating transgenic Arabidopsis pollen compared with wild-type plants. These results demonstrate that PwTUA1 up-regulated by calcium ions and boron contributes to pollen tube elongation by altering the distribution of alpha-tubulin and regulating the deposition of pollen cell wall components during the process of tube growth. The possible role of PwTUA1 in microtubule dynamics and organization was discussed.
Figures


Similar articles
-
PwHAP5, a CCAAT-binding transcription factor, interacts with PwFKBP12 and plays a role in pollen tube growth orientation in Picea wilsonii.J Exp Bot. 2011 Oct;62(14):4805-17. doi: 10.1093/jxb/err120. Epub 2011 Jul 22. J Exp Bot. 2011. PMID: 21784992 Free PMC article.
-
The block of intracellular calcium release affects the pollen tube development of Picea wilsonii by changing the deposition of cell wall components.Protoplasma. 2008;233(1-2):39-49. doi: 10.1007/s00709-008-0310-2. Epub 2008 Aug 26. Protoplasma. 2008. PMID: 18726547
-
γ-Aminobutyric acid (GABA) homeostasis regulates pollen germination and polarized growth in Picea wilsonii.Planta. 2013 Nov;238(5):831-43. doi: 10.1007/s00425-013-1938-5. Epub 2013 Jul 31. Planta. 2013. PMID: 23900837
-
The regulation of vesicle trafficking by small GTPases and phospholipids during pollen tube growth.Sex Plant Reprod. 2010 Jun;23(2):87-93. doi: 10.1007/s00497-009-0118-z. Epub 2009 Nov 7. Sex Plant Reprod. 2010. PMID: 20490965 Review.
-
Calcium: A Critical Factor in Pollen Germination and Tube Elongation.Int J Mol Sci. 2019 Jan 19;20(2):420. doi: 10.3390/ijms20020420. Int J Mol Sci. 2019. PMID: 30669423 Free PMC article. Review.
Cited by
-
Changes in the accumulation of alpha- and beta-tubulin during bud development in Vitis vinifera L.Planta. 2010 Jan;231(2):277-91. doi: 10.1007/s00425-009-1053-9. Epub 2009 Nov 13. Planta. 2010. PMID: 19911193
-
PwHAP5, a CCAAT-binding transcription factor, interacts with PwFKBP12 and plays a role in pollen tube growth orientation in Picea wilsonii.J Exp Bot. 2011 Oct;62(14):4805-17. doi: 10.1093/jxb/err120. Epub 2011 Jul 22. J Exp Bot. 2011. PMID: 21784992 Free PMC article.
-
Importance of organellar proteins, protein translocation and vesicle transport routes for pollen development and function.Plant Reprod. 2016 Jun;29(1-2):53-65. doi: 10.1007/s00497-016-0274-x. Epub 2016 Feb 13. Plant Reprod. 2016. PMID: 26874709 Review.
-
Establishment of the male germline and sperm cell movement during pollen germination and tube growth in maize.Plant Signal Behav. 2010 Jul;5(7):885-9. doi: 10.4161/psb.5.7.12033. Epub 2010 Jul 1. Plant Signal Behav. 2010. PMID: 20505353 Free PMC article.
-
Fitness costs linked to dinitroaniline resistance mutation in Setaria.Heredity (Edinb). 2011 Jul;107(1):80-6. doi: 10.1038/hdy.2010.169. Epub 2011 Jan 19. Heredity (Edinb). 2011. PMID: 21245896 Free PMC article.
References
-
- Abe T, Thitamadee S, Hashimoto T. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant and Cell Physiology. 2004;45:211–220. - PubMed
-
- Åström H, Sorri O, Raudaskoski M. Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sexual Plant Reproduction. 1995;8:61–69.
-
- Cai G, Del CC, Romagnoli S, Cresti M. Pollen cytoskeleton during germination and tube growth. Current Science. 2005;89:1853–1860.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

