Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells
- PMID: 19454660
- PMCID: PMC3082361
- DOI: 10.4049/jimmunol.0804211
Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells
Abstract
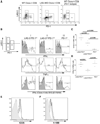
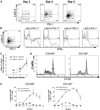
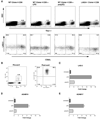
Lymphocyte Activation Gene-3 (LAG-3) is a transmembrane protein that binds MHC class II, enhances regulatory T cell activity, and negatively regulates cellular proliferation, activation, and homeostasis of T cells. Programmed Death 1 (PD-1) also negatively regulates T cell function. LAG-3 and PD-1 are both transiently expressed on CD8 T cells that have been stimulated during acute activation. However, both LAG-3 and PD-1 remain on CD8 T cells at high levels after stimulation within tolerizing environments. Our previous data demonstrated that blockade of either LAG-3 or PD-1 using mAb therapy in combination with vaccination restores the function of tolerized Ag-specific CD8 T cells in models of self and tumor tolerance. It is unclear whether tolerized CD8 T cells coexpress PD-1 and LAG-3 or whether PD-1 and LAG-3 mark functionally distinct populations of CD8 T cells. In this study, we describe three populations of CD8 T cells activated under tolerizing conditions based on LAG-3 and PD-1 staining, each with distinct phenotypic and functional characteristics. From a mechanistic perspective, both Ag concentration and proinflammatory signals control the expression of LAG-3 and PD-1 phenotypes on CD8 T cells under activating and tolerizing conditions. These results imply that signaling through the PD-1 and LAG-3 pathways have distinct functional consequences to CD8 T cells under tolerizing conditions and manipulation of both Ag and cytokine signaling can influence CD8 tolerance through LAG-3 and PD-1.
Figures



References
-
- Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005;35:2081–2088. - PubMed
-
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. - PubMed
-
- Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 2004;172:5450–5455. - PubMed
-
- Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur. J. Immunol. 2003;33:970–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

