Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion
- PMID: 19454664
- PMCID: PMC2923544
- DOI: 10.4049/jimmunol.0800997
Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion
Abstract
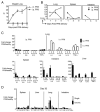
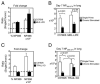
The failure of CD8(+) T cells to respond to chronic infection has been termed "exhaustion" and describes the condition in which CD8(+) T cells exhibit reduced differentiation, proliferation, and effector function. CD8(+) T cell exhaustion has been extensively studied in the murine model of chronic infection, lymphocytic choriomeningitis virus (LCMV). Although LCMV-based studies have yielded many interesting findings, they have not allowed for discrimination between the roles of cytokine- and Ag-driven exhaustion. We have created a system of chronic Ag stimulation using murine influenza A virus that leads to exhaustion and functional disability of virus-specific CD8(+) T cells, in the absence of high viral titers, sustained proinflammatory cytokine production and lymphocyte infection. Our findings show that Ag alone is sufficient to drive CD8(+) T cell impairment, that Ag-driven loss of virus-specific CD8(+) T cells is TRAIL mediated, and that removal of Ag reverses exhaustion. Although programmed death 1 was up-regulated on chronic Ag-stimulated CD8(+) T cells, it played no role in the exhaustion. These findings provide a novel insight into the mechanisms that control functional exhaustion of CD8(+) T cells in chronic infection.
Figures






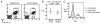

References
-
- McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 1983;309:13–17. - PubMed
-
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

