Cooperation between molecular targets of costimulation in promoting T cell persistence and tumor regression
- PMID: 19454669
- PMCID: PMC2748393
- DOI: 10.4049/jimmunol.0804387
Cooperation between molecular targets of costimulation in promoting T cell persistence and tumor regression
Abstract
Costimulation regulates multiple cellular processes of T cells inducing proliferation, expansion, and survival. The molecular targets of costimulation might then be useful to augment T cell activities. Two defined targets of costimulatory signals in primary T cells are the anti-apoptotic bcl-2 family molecule Bcl-x(L), and survivin, an inhibitor of apoptosis family member that might regulate both cell division and survival. However, the relative importance of, and relationship between, these molecules in primary T cells is not clear. To understand whether they have overlapping or cooperative functions, we used retrovirus-mediated transduction to introduce Bcl-x(L) and survivin separately, or together linked by a 2A picornavirus self-cleaving peptide, into Ag-responding CD8(+) T cells. We found that CD8(+) effector T cells expressing both Bcl-x(L) and survivin strongly expanded at an early stage and had a long-term survival advantage over cells transduced with either molecule alone. In vivo, with response to tumor-expressed Ag following adoptive T cell transfer, Ag-reactive CD8(+) T cells expressing both Bcl-x(L) and survivin displayed greatly enhanced tumor protective activity compared with CD8(+) T cells expressing either molecule introduced separately. These results indicate that Bcl-x(L) and survivin can critically contribute in a cooperative, nonredundant manner to augment the accumulation and persistence of CD8(+) T cells following encounter with Ag. The data provide new insights into why costimulatory signals might need to be sustained over time and suggest a potential novel approach to augment cellular immunotherapy for cancer.
Figures



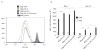

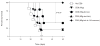

Similar articles
-
OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen.J Immunol. 2005 Sep 15;175(6):3534-41. doi: 10.4049/jimmunol.175.6.3534. J Immunol. 2005. PMID: 16148096
-
The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation.J Immunol. 2010 Dec 1;185(11):6670-8. doi: 10.4049/jimmunol.1000159. Epub 2010 Nov 3. J Immunol. 2010. PMID: 21048108
-
Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy.PLoS One. 2013;8(4):e60031. doi: 10.1371/journal.pone.0060031. Epub 2013 Apr 1. PLoS One. 2013. PMID: 23560068 Free PMC article.
-
[Research advances on inhibitor of apoptosis, survivin].Ai Zheng. 2003 Jul;22(7):771-4. Ai Zheng. 2003. PMID: 12866973 Review. Chinese.
-
Survivin: a protein with dual roles in mitosis and apoptosis.Int Rev Cytol. 2005;247:35-88. doi: 10.1016/S0074-7696(05)47002-3. Int Rev Cytol. 2005. PMID: 16344111 Review.
Cited by
-
FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development.Arthritis Res Ther. 2010;12(2):R66. doi: 10.1186/ar2983. Epub 2010 Apr 12. Arthritis Res Ther. 2010. PMID: 20384988 Free PMC article.
-
C-Myc regulation by costimulatory signals modulates the generation of CD8+ memory T cells during viral infection.Open Biol. 2016 Jan;6(1):150208. doi: 10.1098/rsob.150208. Open Biol. 2016. PMID: 26791245 Free PMC article.
-
IFN-γ upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells.PLoS One. 2010 Nov 22;5(11):e14076. doi: 10.1371/journal.pone.0014076. PLoS One. 2010. PMID: 21124930 Free PMC article.
-
CAR T-cells for colorectal cancer immunotherapy: Ready to go?Front Immunol. 2022 Nov 15;13:978195. doi: 10.3389/fimmu.2022.978195. eCollection 2022. Front Immunol. 2022. PMID: 36458008 Free PMC article. Review.
-
Melanoma Immunotherapy in Mice Using Genetically Engineered Pluripotent Stem Cells.Cell Transplant. 2016;25(5):811-27. doi: 10.3727/096368916X690467. Epub 2016 Jan 15. Cell Transplant. 2016. PMID: 26777320 Free PMC article.
References
-
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. - PubMed
-
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. - PubMed
-
- Yang Y, Liu XK, Nguyen T, Bishop C, Graf D, Dong C. Characterization of B7S3 as a novel negative regulator of T cells. J Immunol. 2007;178:3661–3667. - PubMed
-
- Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

