Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines
- PMID: 19454718
- PMCID: PMC2805849
- DOI: 10.4049/jimmunol.0802971
Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines
Abstract
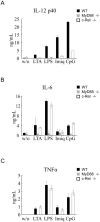
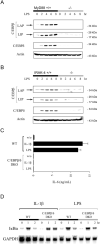
TLR stimulation triggers a signaling pathway via MyD88 and IL-1R-associated kinase 4 that is essential for proinflammatory cytokine induction. Although NF-kappaB has been shown to be one of the key transcriptional regulators of these cytokines, evidence suggests that other factors may also be important. In this study, we showed that MyD88-deficient macrophages have defective c-Rel activation, which has been linked to IL-12p40 induction, but not IL-6 or TNF-alpha. We also investigated other transcription factors and showed that C/EBPbeta and C/EBPdelta expression was limited in MyD88- or IL-1R-associated kinase 4-deficient macrophages treated with LPS. Importantly, the absence of both C/EBPbeta and C/EBPdelta resulted in the impaired induction of proinflammatory cytokines stimulated by several TLR ligands. Our results identify c-Rel and C/EBPbeta/delta as important transcription factors in a MyD88-dependent pathway that regulate the induction of proinflammatory cytokines.
Figures





Similar articles
-
Functional cooperation of simian virus 40 promoter factor 1 and CCAAT/enhancer-binding protein beta and delta in lipopolysaccharide-induced gene activation of IL-10 in mouse macrophages.J Immunol. 2003 Jul 15;171(2):821-8. doi: 10.4049/jimmunol.171.2.821. J Immunol. 2003. PMID: 12847250
-
C5a-regulated CCAAT/enhancer-binding proteins β and δ are essential in Fcγ receptor-mediated inflammatory cytokine and chemokine production in macrophages.J Biol Chem. 2012 Jan 27;287(5):3217-30. doi: 10.1074/jbc.M111.280834. Epub 2011 Dec 6. J Biol Chem. 2012. PMID: 22147692 Free PMC article.
-
Lipopolysaccharide-induced transcriptional activation of interleukin-10 is mediated by MAPK- and NF-kappaB-induced CCAAT/enhancer-binding protein delta in mouse macrophages.Cell Signal. 2006 Sep;18(9):1492-500. doi: 10.1016/j.cellsig.2005.12.001. Epub 2006 Jan 18. Cell Signal. 2006. PMID: 16413748
-
Role of transcriptional factors Sp1, c-Rel, and c-Jun in LPS-induced C/EBPdelta gene expression of mouse macrophages.Cell Mol Life Sci. 2007 Dec;64(24):3282-94. doi: 10.1007/s00018-007-7375-5. Cell Mol Life Sci. 2007. PMID: 17965828 Free PMC article.
-
Differential control of Mincle-dependent cord factor recognition and macrophage responses by the transcription factors C/EBPβ and HIF1α.J Immunol. 2014 Oct 1;193(7):3664-75. doi: 10.4049/jimmunol.1301593. Epub 2014 Aug 25. J Immunol. 2014. PMID: 25156364
Cited by
-
CCAAT/enhancer-binding protein δ is a critical mediator of lipopolysaccharide-induced acute lung injury.Am J Pathol. 2013 Feb;182(2):420-30. doi: 10.1016/j.ajpath.2012.10.013. Epub 2012 Nov 21. Am J Pathol. 2013. PMID: 23177475 Free PMC article.
-
Macrophages and angiogenesis in rheumatic diseases.Vasc Cell. 2013 Jun 1;5(1):11. doi: 10.1186/2045-824X-5-11. Vasc Cell. 2013. PMID: 23725043 Free PMC article.
-
High-Throughput Screening for CEBPD-Modulating Compounds in THP-1-Derived Reporter Macrophages Identifies Anti-Inflammatory HDAC and BET Inhibitors.Int J Mol Sci. 2021 Mar 16;22(6):3022. doi: 10.3390/ijms22063022. Int J Mol Sci. 2021. PMID: 33809617 Free PMC article.
-
Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage.FASEB J. 2010 Jul;24(7):2178-90. doi: 10.1096/fj.09-136895. Epub 2010 Feb 24. FASEB J. 2010. PMID: 20181940 Free PMC article.
-
C/EBPδ deficiency sensitizes mice to ionizing radiation-induced hematopoietic and intestinal injury.PLoS One. 2014 Apr 18;9(4):e94967. doi: 10.1371/journal.pone.0094967. eCollection 2014. PLoS One. 2014. PMID: 24747529 Free PMC article.
References
-
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. - PubMed
-
- Watts C, Zaru R, Prescott AR, Wallin RP, West MA. Proximal effects of Toll-like receptor activation in dendritic cells. Curr Opin Immunol. 2007;19:73–78. - PubMed
-
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

