Hepatic retinol secretion and storage are altered by dietary CLA: common and distinct actions of CLA c9,t11 and t10,c12 isomers
- PMID: 19454764
- PMCID: PMC2759834
- DOI: 10.1194/jlr.M900054-JLR200
Hepatic retinol secretion and storage are altered by dietary CLA: common and distinct actions of CLA c9,t11 and t10,c12 isomers
Abstract
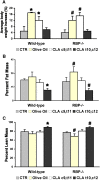
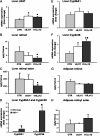
Conjugated linoleic acid (CLA) is a polyunsaturated fatty acid obtained from ruminant products. Previous studies in rats and pigs showed that a dietary equimolar mixture of c9,t11 and t10,c12 CLA isomers induces changes in serum and tissue levels of retinoids (vitamin A derivatives). However, the mechanism(s) responsible for these actions remain(s) unexplored. Given the numerous crucial biological functions regulated by retinoids, it is key to establish whether the perturbations in retinoid metabolism induced by dietary CLA mediate some of the beneficial effects associated with intake of this fatty acid or, rather, have adverse consequences on health. To address this important biological question, we began to explore the mechanisms through which dietary CLA alters retinoid metabolism. By using enriched preparations of CLA c9,t11 or CLA t10,c12, we uncoupled the effects of these two CLA isomers on retinoid metabolism. Specifically, we show that both isomers induce hepatic retinyl ester accumulation. However, only CLA t10,c12 enhances hepatic retinol secretion, resulting in increased serum levels of retinol and its specific carrier, retinol-binding protein (RBP). Dietary CLA t10,c12 also redistributes retinoids from the hepatic stores toward the adipose tissue and possibly stimulates hepatic retinoid oxidation. Using mice lacking RBP, we also demonstrate that this key protein in retinoid metabolism mediates hepatic retinol secretion and its redistribution toward fat tissue induced by CLA t10,c12 supplementation.
Figures


Similar articles
-
A single dose of c9,t11 or t10,c12 conjugated linoleic acid isomers perturbs vitamin A metabolism in mice.Nutr Res. 2011 Nov;31(11):855-62. doi: 10.1016/j.nutres.2011.09.013. Nutr Res. 2011. PMID: 22118757 Free PMC article.
-
Trans-10,cis-12 CLA increases liver and decreases adipose tissue lipids in mice: possible roles of specific lipid metabolism genes.Lipids. 2003 May;38(5):497-504. doi: 10.1007/s11745-003-1090-0. Lipids. 2003. PMID: 12880104
-
Effect of CLA isomers and their mixture on aging C57Bl/6J mice.Eur J Nutr. 2009 Oct;48(7):409-18. doi: 10.1007/s00394-009-0029-7. Epub 2009 May 8. Eur J Nutr. 2009. PMID: 19424653
-
Conjugated linoleic acid isomers and cancer.J Nutr. 2007 Dec;137(12):2599-607. doi: 10.1093/jn/137.12.2599. J Nutr. 2007. PMID: 18029471 Review.
-
Dietary conjugated linoleic acid and body composition.Am J Clin Nutr. 2004 Jun;79(6 Suppl):1153S-1158S. doi: 10.1093/ajcn/79.6.1153S. Am J Clin Nutr. 2004. PMID: 15159250 Review.
Cited by
-
Insights into the molecular mechanisms of the anti-atherogenic actions of flavonoids in normal and obese mice.PLoS One. 2011;6(10):e24634. doi: 10.1371/journal.pone.0024634. Epub 2011 Oct 10. PLoS One. 2011. PMID: 22016761 Free PMC article.
-
Changes in Intestinal Microbiota Are Associated with Islet Function in a Mouse Model of Dietary Vitamin A Deficiency.J Diabetes Res. 2020 Jan 21;2020:2354108. doi: 10.1155/2020/2354108. eCollection 2020. J Diabetes Res. 2020. PMID: 32064275 Free PMC article.
-
Metabolic interactions between vitamin A and conjugated linoleic acid.Nutrients. 2014 Mar 24;6(3):1262-72. doi: 10.3390/nu6031262. Nutrients. 2014. PMID: 24667133 Free PMC article. Review.
-
Amphiphilic cytokine traps remodel marrow adipose tissue for hematopoietic microenvironment amelioration.Bioact Mater. 2024 Sep 3;42:226-240. doi: 10.1016/j.bioactmat.2024.08.032. eCollection 2024 Dec. Bioact Mater. 2024. PMID: 39285915 Free PMC article.
-
Intact vitamin A transport is critical for cold-mediated adipose tissue browning and thermogenesis.Mol Metab. 2020 Dec;42:101088. doi: 10.1016/j.molmet.2020.101088. Epub 2020 Sep 28. Mol Metab. 2020. PMID: 32992038 Free PMC article.
References
-
- Goodman D. S. 1984. Vitamin A and retinoids in health and disease. N. Engl. J. Med. 310: 1023–1031. - PubMed
-
- Napoli J. L. 1996. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin. Immunol. Immunopathol. 80: S52–S62. - PubMed
-
- Blomhoff R., Blomhoff H. K. 2006. Overview of retinoid metabolism and function. J. Neurobiol. 66: 606–630. - PubMed
-
- Sporn M. B., Roberts A. B., Goodman D. S. 1994. The Retinoids: Biology, Chemistry, and Medicine. 2nd ed Raven Press, New York.
-
- Vogel S., Gamble M. V., Blaner W. S. 1999. Biosynthesis, absorption, metabolism and transport of retinoids. Handbook of Experimental Pharmacology. Retinoids. The Biochemical and Molecular Basis of Vitamin A and Retinoid Action. Nau H., Blaner W. S., Springer Verlag Publishing, Heidelberg, Germany: 31–95.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

