E. coli NfsA: an alternative nitroreductase for prodrug activation gene therapy in combination with CB1954
- PMID: 19455141
- PMCID: PMC2690450
- DOI: 10.1038/sj.bjc.6605094
E. coli NfsA: an alternative nitroreductase for prodrug activation gene therapy in combination with CB1954
Abstract
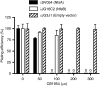
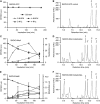
Prodrug activation gene therapy is a developing approach to cancer treatment, whereby prodrug-activating enzymes are expressed in tumour cells. After administration of a non-toxic prodrug, its conversion to cytotoxic metabolites directly kills tumour cells expressing the activating enzyme, whereas the local spread of activated metabolites can kill nearby cells lacking the enzyme (bystander cell killing). One promising combination that has entered clinical trials uses the nitroreductase NfsB from Escherichia coli to activate the prodrug, CB1954, to a potent bifunctional alkylating agent. NfsA, the major E. coli nitroreductase, has greater activity with nitrofuran antibiotics, but it has not been compared in the past with NfsB for the activation of CB1954. We show superior in vitro kinetics of CB1954 activation by NfsA using the NADPH cofactor, and show that the expression of NfsA in bacterial or human cells results in a 3.5- to 8-fold greater sensitivity to CB1954, relative to NfsB. Although NfsB reduces either the 2-NO(2) or 4-NO(2) positions of CB1954 in an equimolar ratio, we show that NfsA preferentially reduces the 2-NO(2) group, which leads to a greater bystander effect with cells expressing NfsA than with NfsB. NfsA is also more effective than NfsB for cell sensitisation to nitrofurans and to a selection of alternative, dinitrobenzamide mustard (DNBM) prodrugs.
Figures



References
-
- Anlezark GM, Melton RG, Sherwood RF, Coles B, Friedlos F, Knox RJ (1992) The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)–I Purification and properties of a nitroreductase enzyme from Escherichia coli–a potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT). Biochem Pharmacol 44: 2289–2295 - PubMed
-
- Anlezark GM, Melton RG, Sherwood RF, Wilson WR, Denny WA, Palmer BD, Knox RJ, Friedlos F, Williams A (1995) Bioactivation of dinitrobenzamide mustards by an E coli B nitroreductase. Biochem Pharmacol 50: 609–618 - PubMed
-
- Anlezark GM, Vaughan T, Fashola-Stone E, Paul Michael N, Murdoch H, Sims MA, Stubbs S, Wigley S, Minton NP (2002) Bacillus amyloliquefaciens orthologue of Bacillus subtilis ywrO encodes a nitroreductase enzyme which activates the prodrug CB 1954. Microbiology 148: 297–306 - PubMed
-
- Atwell GJ, Yang S, Pruijn FB, Pullen SM, Hogg A, Patterson AV, Wilson WR, Denny WA (2007) Synthesis and structure-activity relationships for 2,4-Dinitrobenzamide-5-mustards as prodrugs for the Escherichia coli nfsB nitroreductase in gene therapy. J Med Chem 50(6): 1197–212 - PubMed
-
- Bailey SM, Knox RJ, Hobbs SM, Jenkins TC, Mauger AB, Melton RG, Burke PJ, Connors TA, Hart IR (1996) Investigation of alternative prodrugs for use with E coli nitroreductase in ‘suicide gene’ approaches to cancer therapy. Gene Ther 3: 1143–1150 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

